Facile method of preparation of PbS films for NO2 detection
S. T. Navale,
D. K. Bandgar,
M. A. Chougule and
V. B. Patil*
Functional Materials Research Laboratory, School of Physical Sciences, Solapur University, Solapur-413255, MS, India. E-mail: drvbpatil@gmail.com; Tel: +91 2172744771 ext. 202
First published on 16th December 2014
Abstract
A simple and inexpensive chemical bath deposition method was employed for the preparation of lead sulfide (PbS) thin films. Thin films of PbS were characterized using XRD, SEM, TEM, SAED, contact angle and two probe techniques. The gas sensing properties of PbS thin films (t = 0.443 μm) have been investigated for H2S, NH3, C2H5OH, CH3OH, Cl2 and NO2. PbS thin films have been found to be more highly sensitive and selective towards oxidizing NO2 gas than the other test gases. The PbS sensor exhibits a maximum response of 74% towards 100 ppm of NO2 gas with a response time of 20 s. PbS thin films are able to detect up to 5 ppm NO2 concentration at room temperature with excellent reproducibility and stability. The sensing mechanism of PbS thin films towards NO2 gas is also discussed. The interaction of NO2 gas with PbS film was also investigated using impedance spectroscopy.
1 Introduction
In recent years, a tremendous amount of studies have been carried out on various materials as gas sensors due to the increasing concerns about environmental and health protection as well as accidental leakages of explosive gases. Nowadays, current research in the field of gas sensors has been focused on the development of low cost and highly sensitive efficient sensor devices for monitoring of hazardous, corrosive, toxic and flammable gases. Nitrogen dioxide, NO2, is one of the toxic, harmful and foul-smelling gases and is created during the several processes such as the high temperature combustion of coal, combustion of gasoline in internal combustion engines, home heaters, natural gas or oil in power plants, exhaust of furnaces, chemical production and also by the automobile engines.1–3 Nitrogen dioxide is a main cause of acid rain and formation of photochemical smog as well as NO2 is involved in the depletion of ozone in the stratosphere.2,4 Therefore, it is necessary to develop efficient devices which can sense toxic and corrosive NO2 gas at low level (∼ppm). Over the past few years, use of nanostructured inorganic semiconductors in a sensing material has attracted much attention of researchers because of their unique electrical and optical properties. Furthermore their low cost, ease of production, simplicity and capability of detecting large number of toxic as well as flammable gases under different conditions makes them suitable candidate in gas sensor field.5,6 It is well known that, inorganic semiconductor based gas sensors involve a catalytic reaction (reduction or oxidation) of the target gas on the surface of the sensor.Lead sulfide (PbS) is a typical direct narrow band gap IV–VI compound semiconductor, which possess special optoelectronic properties and found wide applications in optical communication apparatus, radioactivity detector and optical information storage.7–9 Furthermore, PbS consists of large excitation Bohr radius (18 nm), which results in good quantum confinement of both holes and electrons in nanosized structure. Therefore, according to the effective mass model the value of the band gap can be easily controlled by modifying the particle shape as well as the size.
Therefore, the preparation of PbS thin film interests is growing in developing new processing routes. Currently, different techniques are in use for the fabrication of desirably shaped and structured thin films including chemical and physical methods. Chemical methods are economic and inexpensive as well as beautiful thin film structures can be obtained with them. Furthermore, physical methods are suitable for the fabrication of uniform and high quality thin films. But, these methods are expensive and highly energy consuming.10 In recent years, various techniques have been used to deposit PbS thin films including microwave assisted chemical bath deposition,11 successive ionic layer adsorption and reaction (SILAR) technique,12 hydro chemical deposition,13 atomic layer epitaxial process,14 pulse electrodeposition,15 chemical bath deposition16 and solid-vapor deposition.17
Among these various techniques, chemical bath deposition method also called as chemical solution deposition technique have a several advantages and is widely used for thin film deposition because of its low temperature operating condition, low cost, no requirement of sophisticated instruments, freedom to deposit materials on a variety of substances suitability for large scale deposition areas, ability of tuning thin film properties easily by adjusting and controlling the deposition experimental parameters and non-polluting properties.18–20 Normally, lead sulfide is used as an optical and a semiconductor material as well as photoconductive devices as detectors.21 Very few reports are available on the application of semiconducting lead sulfide as a gas sensor. Shimizu et al.22 reported SO2 gas sensing properties of Pb1−xCdxS (x = 0.1, 0.2) system and the metal-mono sulfide based (NiS, CdS, SnS and PbS) solid electrolyte sensor elements operating in between 300–400 °C. Fu23 reported, the NO2 and NH3 gas sensing properties of lead sulfide prepared by precipitation method at various operating temperatures with 5 V operating voltage. Markov et al.24 reported the NO and NO2 gas sensing properties of iodide doped lead sulfide films by chemical deposition method. While, Karami et al.25 reported the ammonia sensing properties of lead sulfide nanostructures prepared by chemical precipitation method. However, so far there is no report available on the preparation of lead sulfide by chemical bath deposition method and their room temperature NO2 gas sensing properties at low concentration (5–100 ppm).
In the present paper, we demonstrate gas sensing properties of nanocrystalline PbS thin films prepared by simple and low cost chemical bath deposition method to develop a reliable, sensitive and low cost chemical sensor for monitoring nitrogen dioxide. Thin films of PbS have been characterized using XRD, FESEM, TEM, SAED and two probe techniques. Various reducing and oxidizing gases such as NH3, H2S, CH3OH, C2H5OH, NO2 and Cl2 were used as target gases. The systematic gas sensing performance of PbS thin films was carried out at room temperature and explored. Also the plausible sensing mechanism between PbS thin film and NO2 gas is discussed using impedance spectroscopy.
2 Experimental details
2.1 Materials
Lead acetate (AR grade, Sd Fine Chem. Ltd., India), thiourea (AR grade, Sd Fine Chem. Ltd., India), ammonia (30% AR grade, Sd Fine Chem. Ltd., India), hydrochloric acid (AR grade, Sd Fine Chem. Ltd., India).2.2 Preparation of nanocrystalline PbS thin films
Thin films of lead sulfide were deposited on commercially available glass slides of the dimensions of 7.5 cm × 2.5 cm by inexpensive chemical bath deposition method. For the thin film formation, 0.005 M of lead acetate was complexed with 0.15 M of thiourea [CS (NH2)2] with vigorous stirring. The glass substrates were vertically immersed in magnetically stirred bath solution. The pH of the solution was maintained at 10–11 by adding ammonia as a complexing agent. The deposition was carried out at temperature of 60 °C for 40 min. After a 40 min deposition time, the glass substrates were removed from bath and washed thoroughly with distilled water and dried in air. It was observed that, chemical bath deposited PbS thin films are uniform, dark brown colored and well adherent to the glass substrate.2.3 Characterization techniques
The structural properties of the prepared PbS thin films were carried out using X-ray diffractometer (Model: PW-3710, Holland) at step width of 0.02° min−1 in 2θ range of 10–80° with CuKα radiation (λ = 1.5406 Å). The surface morphology of the prepared samples were analyzed using scanning electron microscopy (SEM, Model: JEOL JSM 6360) operating at 20 kV. The Transmission electron microscopy and selected area electron diffraction of PbS film was carried out using Hitachi Model H-800 transmission electron microscopy. DC electrical conductivity and thermoelectric power measurement was carried out using two-point probe technique. The thickness of the as deposited films was measured using an Ambious XP-1 surface profilometer and it is found to be 0.449 μm.Gas sensing characteristics of the prepared films was measured using custom fabricated room temperature gas sensing measurement unit described elsewhere.6 The electrical resistance of a sensor films was measured in gas and in air atmosphere. In order to measure resistance of the films two silver electrodes separated by 10 mm each other were deposited on PbS thin film using silver paint and dried in air atmosphere. Various reducing viz. H2S, NH3, CH3OH, C2H5OH, and oxidizing gases as NO2 and Cl2 were used as test gases. All these test gases were commercially procured from Space Cryogases Pvt. Ltd. Mumbai, India. For the measurements of gas sensing properties, sensor films were mounted in an airtight stainless steel chamber having volume of 250 cm3. Desired concentration of a various test gases in the airtight stainless steel chamber was attained by introducing a measured quantity of known gas using a syringe. The change in resistance value of the sensor films were recorded using a Keithley 6514 System Electrometer controlled by a computer. Once resistance stability was achieved, sensor recovery was measured by opening the lid of the chamber to air atmosphere. All the gas sensing measurements on PbS thin films were carried out at room temperature. It was observed that, on exposure to oxidizing gases (NO2 and Cl2) the sample resistance was found to be decreasing, while on exposure to reducing gases (NH3, CH3OH, C2H5OH and H2S) the sample resistance increases, as expected for p-type nature of as synthesized PbS.
The response (S) was calculated using the relation,
| S (%) = |Ra − Rg|/Ra × 100 | (1) |
Impedance studies on PbS thin film in air and in gas environment was carried out using Wayne Kerr make precision impedance analyzer (Model: 6500-B) in the frequency range of 20 Hz to 10 MHz. All the measurements were carried out at room temperature (311 K).
3 Results and discussion
3.1 PbS thin film formation mechanism
It is well known that, in chemical bath deposition method film formation takes place when the product of concentrations of cations and anions exceeds the solubility product. In other words, when solution supersaturates then ionic product of cation and anion is greater than the solubility product.26 Here, lead sulfide is formed when the ionic product of Pb2+ and S2− ions exceeds the solubility product of PbS. Therefore, the concentration of lead and sulphur ions controlled very carefully during the growth. The formation of PbS may involve sequential reactions at the substrate surface and is enlightened in the following steps. When lead acetate [Pb(CH3COO)2] dissolved in aqueous solution then releases Pb2+ ions. Dissociations of thiourea [CS(NH2)2] occur due to the addition of ammonia resulting into formation of SH− ions, which then reacts with hydroxide species to give S2− anions. Finally, PbS thin film is formed when Pb2+ cations are adsorbed on the substrate and combines with S2− anions. The overall growth occurs by ion-by-ion process on the substrate and the corresponding reaction mechanism is as follows:27| Pb(CH3COO)2 → Pb2+ + 2CH3COO− | (2) |
 | (3) |
| SH− + OH− → S2− + H2O | (4) |
| Pb2+ + S2− → PbS | (5) |
3.2 X-ray diffraction analysis
X-ray diffraction pattern of PbS thin film deposited on glass substrate by chemical bath deposition method is shown in Fig. 1(a). In the XRD pattern of PbS, the observed broad hump is due to amorphous glass substrate.26 Nanocrystalline PbS thin film shows prominent diffraction peaks at 2θ = 26.60°, 30.70°, 43.62°, 51.42°, 53.90°, 62.85°, 69.15°, 71.30° and 79.30° are assigned to the scattering from (111), (200), (220), (311), (222), (400), (311), (420) and (422) planes of the cubic PbS structure respectively, which are well matched with the reported values for PbS [JCPDS card no. 78-1899]. The calculated lattice constant is found to be 5.88 Å and which is close to the JCPDS file [a = b = c = 5.92 Å]. A preferred orientation of the deposited PbS thin film is observed along (1 1 1) direction. Therefore, the crystallite size for PbS thin film was estimated using the full width at half maximum (FWHM) towards (1 1 1) peak from the well known Debye-Scherer's formula [eqn (6)] and it was found to be 38 nm.
D = Kλ/β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ θ
| (6) |
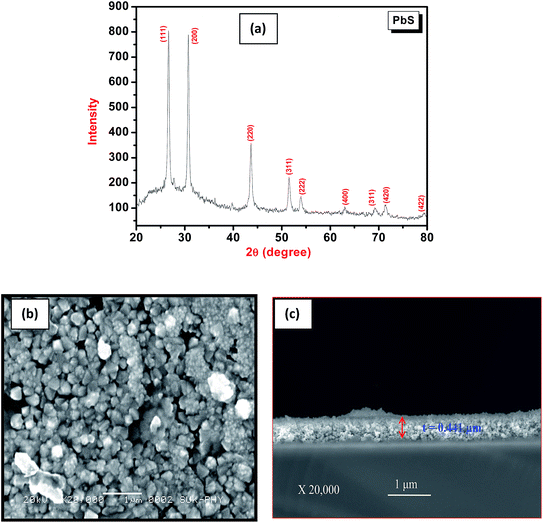 | ||
| Fig. 1 (a) X-ray diffraction pattern of PbS thin film, (b) SEM micrograph of PbS thin film, (c) SEM cross section of PbS thin film. | ||
3.3 SEM analysis
The surface morphology of the nanocrystalline PbS thin film was analyzed using scanning electron microscopy (SEM) and displayed in Fig. 1(b). The deposited film under the optimized conditions was uniformly covered the substrate surface with good adherence. The micrograph of PbS thin film revealed that, a large number of tiny spherical type nanograins are interconnected each other with some pores are available between them.The thickness of chemically deposited PbS thin film also determined by SEM cross section.
Fig. 1(c) shows SEM cross section of PbS film and thickness of PbS is 0.441 μm. It is well match with thickness obtained from thickness profiler.
3.4 TEM and SAED analysis
Transmission electron micrograph (TEM) and selected area electron diffraction (SAED) pattern of PbS film is shown in Fig. 2. The TEM image of PbS film in Fig. 2(a) shows that, interconnected spherical nanoparticles with an average particle size of 41 nm. Selected area electron diffraction pattern of the PbS thin film in Fig. 2(b) showed five clear diffraction rings, which corresponded to the (111), (200), (220), (331) and (222) planes of the cubic PbS respectively. This is in good agreement with the XRD analysis results and indicated that, the PbS nanoparticles are well crystallized. | ||
| Fig. 2 (a) TEM image of PbS thin film, (b) SAED pattern of PbS film, (c) contact angle of PbS thin film. | ||
3.5 Surface wettability study
The as deposited PbS thin film was used in wettability study (water contact angle measurement). When the air is the surrounding medium, the wettability of PbS with water is dependent on the relationship with the interfacial tensions (water/air, water/solid and solid/air). The ratio between these tensions determines the contact angle ‘θ’ between a water droplet on a PbS surface. If the water contact angle is 0° indicate that the surface is completely wet (super hydrophilic), and if the water contact angle is 180° means that the surface is not completely wet (super hydrophobic). The both super-hydrophilic and super-hydrophobic surfaces plays important role in gas sensing applications. Prior to contact angle measurements or treatment, film was rinsed with acetone and de-ionized water. The average contact angle was obtained by measuring for at least five separate drops on each sample surface by delivering de-ionized water with a microsyringe. Measurement of surface water contact angle is inversely proportional to the wettability and can be determined by Young's relation. Fig. 2(c) shows the water contact measurements for PbS thin film.From Fig. 2(c) it is observed that, the PbS thin films are hydrophilic as water contact angle is 75° (less than 90°) means high wettability. This may be due to the strong cohesive force between the water droplet and hydroxide present in the lead sulfide. Due to which the water is attracted rather repelled by the PbS film. This specific property is useful for making intimate contact with electrode surface in gas sensing application. We believed this specific property will tentatively demonstrate the feasibility of PbS film surface useful in material/electrode contact for better performances.
3.6 Electrical transport studies
| σ = σ0(−Ea/KbT) | (7) |
Fig. 3(a) shows the plot of logarithmic conductivity as a function of inverse temperature and from the slope of this curve activation energy was calculated. The activation energy represents the location of trap states below the conduction band and it is 0.7 eV.
3.7 Gas sensing measurement
| Q = SA/SB | (8) |
| Test gas | NH3 | H2S | C2H5OH | Cl2 | CH3OH |
|---|---|---|---|---|---|
| ‘Q’ value | 6.16 | 12.33 | 14.8 | 9.25 | 10.57 |
The inset of Fig. 4(b) shows the variation of gas response (100 ppm NO2) of PbS film for different temperature. Before exposing to NO2 gas, the film with thickness 0.441 μm was allowed to be stable for electrical resistance for 30 min and the stabilized resistance was taken as Ra. Initially, the gas response to 100 ppm of NO2 was measured as a function of operating temperature for film. The sensor response reached maximum at 311 K (gas response = 74%) and then decreased at 323 K (gas response = 67%). The NO2 gas molecules possess sufficient thermal excitation energy to react with adsorbed oxygen species at around 311 K. Therefore at that temperature PbS sensor reaches its maximum response as compared to other operating temperature.
From Fig. 4(c) it is observed that, the response value of PbS thin film sensor increases with increasing concentration of NO2 gas from 5 to 100 ppm at room temperature (311 K). Response of the PbS thin films was found to be linear in 5–100 ppm NO2 range. The responses of PbS thin film on exposure to 5–100 ppm NO2 gas are 31%, 49%, 54%, 67%, 70% and 74% respectively. Lower NO2 gas concentration covers the lower surface of PbS film hence lowers the interactions between NO2 gas and film surface which results into lower response. On the other hand at higher NO2 concentration increases the interactions between NO2 and film surface, due to the higher surface coverage and resulting into higher response. The PbS thin film shows the decrease in resistance value on exposure to oxidizing NO2 gas, which is due to the fact that, electrons of NO2 act as the accepter to the p-type PbS, thus causing a increase in hole concentration in PbS and film resistance decreases. PbS thin film exhibits the maximum response of 74% towards 100 ppm of NO2 gas with very fast response time of 20 second. Such a higher response value is believed to be due to the response of the inorganic semiconductor sensors mainly governed by the interactions between the target gas (herein NO2) and the surface of the sensors. Furthermore, chemical bath deposited PbS thin films are able to detect up low 5 ppm concentration of NO2 gas with very high response of 31%, suggesting their suitability to be used as NO2 sensor. The electrical response of PbS thin film towards different concentrations of NO2 is shown in Fig. 4(d).
Stability performance of PbS thin films were studied for fixed 100 ppm NO2 concentration over the period of 40 days and corresponding results are displayed in Fig. 5(c). Initially PbS thin film exhibits the response of 74%, but it drops (74% to 66%) with time and stable response was attained after 15 days with 89% stability.
Finally, the present work demonstrates competitive results on the highly sensitive and selective room temperature (311 K) NO2 gas sensing properties of PbS thin films prepared by simple and inexpensive chemical bath deposition method. The sensor shows linear variation in response between 5–100 ppm NO2 concentrations with very fast response time and long term stability. Most important, our PbS thin films are strongly adherent to the glass substrate and exhibits excellent reproducibility in response. Such a material with excellent gas sensing properties is suitable for detecting lower concentrations of NO2 at room temperature (311 K).
| 2NO2 + O2− → 2NO3 + 2e− | (9) |
The released electrons from surface states are recombine with the holes in the valance band and therefore film resistance decreases due to the oxidizing nature (electron accepting) of NO2. If PbS thin film is exposed to fresh air atmosphere and NO2 is removed then the recovery process of sensor is achieved. During this reaction, the thickness of accumulation layer decreases (Fig. 6(b)) and which leads to an increase in conductance of the material. The attractive feature of the PbS thin film sensor is its room temperature (311 K) operation, fast response time and the reproducible response characteristics. This may increase shelf life of sensor and makes it reusable.
3.8 Impedance spectroscopy studies
Impedance spectroscopy is a useful technique that has been widely used to distinguish different contributions to sensor response.33,34 In the present study, in order to understand the better response of PbS thin films towards NO2 gas, we have carried out impedance spectroscopy study, before and after exposure of 100 ppm NO2 gas at room temperature (311 K) and the corresponding results are shown in Fig. 7(a) in the form of Cole–Cole plot. The impedance spectra clearly show a single semicircle arc from low frequency to high frequency region. The impedance spectrum was analyzed by using the equivalent circuit shown in Fig. 7(b). It consists of two RC networks, in which R0 is frequency independent resistance, Rb is bulk resistance, Cb is bulk capacitance, Rg is grain boundary resistance and Cg is grain boundary capacitance. Values of various parameters obtained from impedance spectra are given in Table 2. | ||
| Fig. 7 (a) Impedance spectra of PbS film before and after exposure to NO2, (b) equivalent circuit used to interpret the impedance spectroscopy data obtained for PbS. | ||
From Fig. 7(a) it is also seen that, there is very good agreement between the simulated data (shown by solid lines) and experimental data.
On exposure to 100 ppm NO2, both Rb and Rg were seen to decrease while Cb and Cg were found to increase. Based on the obtained results sensing mechanism can be proposed as follows.
PbS grains contains significant fraction of O2− adsorbates at grain boundaries which trap electrons from the grains of PbS results in increasing carrier density and conductivity of inter grain region. On the interaction of PbS film with oxidizing NO2 gas, adsorbed oxygen on the grain boundary of the PbS film is replaced by gaseous species. Due to electron accepting nature of NO2 the resistance of PbS film decreases. Such decreases in resistance on NO2 exposure is mainly contributed by the grain boundaries of the PbS films.
4 Conclusions
The present paper demonstrates room temperature (311 K) gas sensing performance of PbS thin films for different gases viz, Cl2, NH3, H2S, NO2, CH3OH and C2H5OH. The gas sensing studies shows that, the chemical bath deposited PbS thin films are highly sensitive and selective towards NO2 gas with 74% (towards 100 ppm) response. However, PbS sensor exhibits reasonably fast response time of 20 s. A noteworthy feature of the present work is that, the prepared PbS thin film sensors operate at room temperature and are able to detect up low 5 ppm NO2 concentration with reasonable response of 37%. Furthermore, PbS thin films exhibit excellent repeatability with 89% stability. The reliable detection of NO2 gas in low ppm level using PbS thin film makes them attractive candidate for high performance selective NO2 gas sensors.Acknowledgements
Authors (VBP) are grateful to DAE-BRNS, for financial support through the scheme no. 2010/37P/45/BRNS/1442.References
- S. T. Shishiyanu, T. S. Shishiyanu and O. I. Lupan, Novel NO2 gas sensor based on cuprous oxide thin films, Sens. Actuators, B, 2006, 113, 468 CrossRef CAS PubMed.
- S. G. Pawar, S. L. Patil, M. A. Chougule, B. T. Raut, S. A. Pawar, R. N. Mulik and V. B. Patil, Nanocrystalline TiO2 thin films for NH3 monitoring: microstructural and physical characterization, J. Mater. Sci.: Mater. Electron., 2012, 23, 273–279 CrossRef CAS PubMed.
- Y. Shimizu and M. Egashira, Basic aspects and challenges of semiconductor gas sensors, Mater. Res. Bull., 1999, 6, 18 CrossRef.
- S. T. Navale, A. T. Mane, M. A. Chougule, R. D. Sakhare, S. R. Nalage and V. B. Patil, Highly selective and sensitive room temperature NO2 gas sensor based on Polypyrrole thin films, Synth. Met., 2014, 189, 94 CrossRef CAS PubMed.
- Science and technology of chemiresistor gas sensors, ed. D. K. Aswal and S. K. Gupta, Nova Science Publisher, NY, USA, 2007 Search PubMed.
- S. T. Navale, D. K. Bandgar, S. R. Nalage, G. D. Khuspe, M. A. Chougule, Y. D. Kolekar, S. Sen and V. B. Patil, Synthesis of Fe2O3 nanoparticles for nitrogen dioxide gas sensing application, Ceram. Int., 2013, 39, 6453 CrossRef CAS PubMed.
- J. G. Winiarz, L. Zhang, J. Park and P. N. Prasad, Inorganic: organic hybrid nanocomposites for photo refractivity at communication wavelengths, J. Phys. Chem. B, 2002, 106, 967 CrossRef CAS.
- Y. Zhou, H. Itoh, T. Uemura, K. Naka and Y. Chujo, Preparation, optical spectroscopy, and electrochemical studies of novel π-conjugated polymer-protected stable PbS colloidal nanoparticles in a nonaqueous solution, Langmuir, 2002, 18, 5287 CrossRef CAS.
- X. h. Yang, Q. S. Wu, Y. P. Ding and J. k. Liu, Synthesis and optical properties of the semiconductor lead sulfide nanobelts, Bull. Korean Chem. Soc., 2006, 27, 377 CrossRef CAS.
- A. Bauger, J. C. Mutin and J. C. Niepce, Synthesis reaction of metatitanate BaTiO3, J. Mater. Sci., 1983, 18, 3041 CrossRef.
- A. S. Obaid, M. A. Mahdi, Y. Yusof, M. Bououdina and Z. Hassan, Structural and optical properties of nanocrystalline lead sulfide thin films prepared by microwave-assisted chemical bath deposition, Mater. Sci. Semicond. Process., 2013, 16, 971 CrossRef CAS PubMed.
- T. Kanniainen, S. Lindroos, J. Ihanus and M. Leskela, Growth of strongly orientated lead sulfide thin films by successive ionic layer adsorption and reaction (SILAR) technique, Mater. Chem. Phys., 1996, 6, 161 CAS.
- S. I. Sadovnikov, A. I. Gusev and A. A. Rempel, New crystalline phase in thin lead sulfide films, JETP Lett., 2009, 89, 238 CrossRef CAS.
- M. Leskela, L. Niinisto, P. Niemela, E. Nykanen, P. Soininen, M. Tiitta and J. Vahakangas, Preparation of lead sulfide thin films by the atomic layer epitaxy process, Vacuum, 1990, 41, 1457 CrossRef CAS.
- N. R. Mathews, C. C. Angelesa, J. M. A. Corta and A. J. A. Toledo, Physical properties of pulse electrodeposited lead sulfide thin films, Electrochim. Acta, 2013, 99, 84 CrossRef PubMed.
- E. Pentia, L. Pintilie, I. Matei, T. Botila and E. Ozbay, Chemically prepared nanocrystalline PbS thin films, J. Optoelectron. Adv. Mater., 2001, 3, 525 CAS.
- A. S. Obaid, M. A. Mahdi and Z. Hassan, Nanocoral PbS thin film growth by solid-vapor deposition, J. Optoelectron. Adv. Mater., 2012, 6, 422 CAS.
- S. Y. Han, D. H. Lee, Y. J. Chang, S. O. Ryu, T. J. Lee and C. H. Chang, The growth mechanism of nickel oxide thin films by room-temperature chemical bath deposition, J. Electrochem. Soc., 2006, 153, 382 CrossRef PubMed.
- S. T. Navale, A. T. Mane, M. A. Chougule, N. M. Shinde, J. H. Kim and V. B. Patil, Room temperature gas sensing properties of cadmium sulfide thin film based sensor for detection of NO2 gas, RSC Adv., 2014, 4, 44547 RSC.
- A. Kathalingam, N. Ambika, M. R. Kim, J. Elanchezhiyan, Y. S. Chae and J. K. Rhee, Chemical bath deposition and characterization of nanocrystalline ZnO thin films, Mater. Sci.–Pol., 2010, 28, 513 CAS.
- D. Yu, D. Wang, Z. Meng, J. Lu and Y. Qian, Synthesis of closed PbS nanowires with regular geometric morphologies, Mater. Chem. Phys., 2002, 12, 403 CAS.
- Y. Shimizu, M. Okimoto and N. Souda, Solid-state SO2 sensor using a sodium-ionic conductor and a metal sulfide electrode, Int. J. Appl. Ceram. Technol., 2006, 3, 193 CrossRef CAS PubMed.
- T. Fu, Research on gas-sensing properties of lead sulfide-based sensor for detection of NO2 and NH3 at room temperature, Sens. Actuators, B, 2009, 140, 116 CrossRef CAS PubMed.
- V. F. Markov and L. N. Maskaeva, Lead sulfide semiconducting sensing element for nitrogen oxide analysers, J. Anal. Chem., 2001, 56(8), 754 CrossRef CAS.
- H. Karami, M. Ghasemi and S. Matini, Synthesis, characterization and application of lead sulfide nanostructures as ammonia gas sensing agent, Int. J. Electrochem. Sci., 2013, 8, 11661 CAS.
- G. Hodes, Chemical solution deposition of semiconductor films, Marcel Dekker Inc., New York, 2002, p. 5 Search PubMed.
- A. U. Ubale, A. R. Junghare, N. A. Wadibhasme, A. S. Daryapurkar, R. B. Mankar and V. S. Sangawar, Thickness dependent structural, electrical and optical properties of chemically deposited nanoparticles PbS thin Films, Turk. J. Phys., 2007, 31, 279 CAS.
- S. L. Patil, M. A. Chougule, S. G. Pawar, B. T. Raut, S. Sen and V. B. Patil, New process for synthesis of ZnO thin films: Microstructural, optical and electrical characterization, J. Alloys Compd., 2011, 509, 10055 CrossRef CAS PubMed.
- B. T. Raut, S. G. Pawar, M. A. Chougule, S. Sen and V. B. Patil, New process for synthesis of nickel oxide thin films and their characterization, J. Alloys Compd., 2011, 509, 9065 CrossRef CAS PubMed.
- X. Loua, S. Liu, D. Shi and W. Chua, Ethanol sensing characteristics of CdFe2O4 sensor prepared by sol–gel method, Mater. Chem. Phys., 2007, 105, 67 CrossRef PubMed.
- G. D. Khuspe, R. D. Sakhare, S. T. Navale, M. A. Chougule, R. N. Mulik, R. C. Pawar, C. S. Lee and V. B. Patil, Nanostructured SnO2 thin film for NO2 gas sensing applications, Ceram. Int., 2013, 39, 8673 CrossRef CAS PubMed.
- W. Gopel and K. D. Schierbaum, SnO2 sensors: current status and future prospects, Sens. Actuators, B, 1995, 26, 1 CrossRef.
- M. Kaur, S. K. Gupta, C. A. Betty, V. Saxena, V. R. Katti, S. C. Gadkari and J. V. Yakhmi, Detection of reducing gases by SnO2 thin films: an impedance spectroscopy study, Sens. Actuators, B, 2005, 107, 360 CrossRef CAS PubMed.
- S. T. Navale, G. D. Khuspe, M. A. Chougule and V. B. Patil, Polypyrrole, α-Fe2O3 and their hybrid nanocomposite sensor: an impedance spectroscopy study, Org. Electron., 2014, 15, 2159–2167 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |





