Solid phase extraction of heavy metal ions from agricultural samples with the aid of a novel functionalized magnetic metal–organic framework†
Abstract
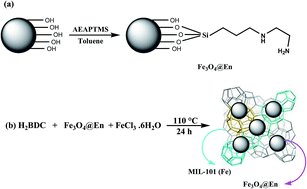
This work describes the synthesis and application of a novel magnetic metal–organic framework (MOF) [(Fe3O4–ethylenediamine)/MIL-101(Fe)] to preconcentrate the trace amounts of Cd(II), Pb(II), Zn(II) and Cr(III) ions and their determination by flame atomic absorption spectrometry. A Box–Behnken design was used to find the parameters affecting the preconcentration procedure through response surface methodology. Three variables, including sorption time, amount of the magnetic sorbent, and sample pH, were selected as affecting factors in sorption step, and four parameters, including type, volume, concentration of the eluent, and elution time, were selected in elution step for the optimization study. The values of the amount of the magnetic sorbent, sorption time, sample pH, type, volume, concentration of the eluent, and elution time were 29 mg, 15 min, 6.1, EDTA + HNO3, 4.2 mL, 0.7 mol L−1 EDTA in 0.07 mol L−1 HNO3 solution, 17.0 min, respectively. The limits of detection (LOD) were 0.15, 0.8, 0.2 and 0.5 ng mL−1 for Cd(II), Pb(II), Zn(II) and Cr(III) ions, respectively. The relative standard deviations (RSD) of the method were less than 7.6% for five separate batch experiments in the determination of 30 μg L−1 of Cd(II), Pb(II), Zn(II) and Cr(III) ions. The sorption capacity of [(Fe3O4–ethylenediamine)/MIL-101(Fe)] was 155 mg g−1 for cadmium, 198 mg g−1 for lead, 164 mg g−1 for zinc and 173 mg g−1 for chromium. Finally, the magnetic MOF nanocomposite was successfully applied to rapidly extract the trace amounts of heavy metal ions in agricultural samples.

 Please wait while we load your content...
Please wait while we load your content...