Cross-linking of poly(vinyl alcohol) with N,N′-methylene bisacrylamide via a radical reaction to prepare pervaporation membranes
Abstract
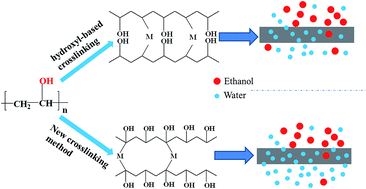
To retain the hydroxyl group of poly(vinyl alcohol) (PVA), ammonium persulfate (APS) was used to initiate the polymerization of hydrogen on the PVA chain. Rather than hydroxyl-based cross-linking, the generated PVA macromolecule radicals were cross-linked with N,N′-methylene bisacrylamide (MBA) to prepare PVA membranes. The PVA membranes were shown to be successfully cross-linked via FT-IR characterization, as well as measurements of swelling degree and gel fraction. With increasing the content of cross-linker, the swelling degree of the membranes decreased, and the gel fraction increased. The pervaporation performance of the membrane was investigated by separating 95 wt% ethanol aqueous solutions. The cross-linked PVA membrane containing 0.5 wt% cross-linker yielded a high permeate water content (99 wt%) and a total flux of 353 g m−2 h−1 at 40 °C. As the feed temperature increased, the total flux increased, while the permeate water content decreased. The cross-linked membrane exhibited good durability over long-term operation.

 Please wait while we load your content...
Please wait while we load your content...