A new chemosensor for Ga3+ detection by fluorescent nitrogen-doped graphitic carbon dots†
Hao Wanga,
Yun Wanga,
Jun Guob,
Ying Sua,
Cheng Suna,
Jie Zhaoa,
Hongmei Luoc,
Xiao Daia and
Guifu Zou*a
aCollege of Physics, Optoelectronics and Energy & Collaborative Innovation Center of Suzhou Nano Science and Technology, Soochow University, Suzhou 215006, P. R. China. E-mail: zouguifu@suda.edu.cn; Fax: +86-512-65228130; Tel: +86-512-65228130
bTesting and Analysis Center, Soochow University, Suzhou, P. R. China
cDepartment of Chemical and Materials Engineering, New Mexico State University, Las Cruces, NM 87003, USA. E-mail: hluo@nmsu.edu
First published on 15th January 2015
Abstract
Highly fluorescent nitrogen-doped graphitic carbon dots (NGCDs) are developed as new label-free chemosensors for gallium ion (Ga3+) detection for the first time. Through the pyrolysis of ammonium citrate in air, NGCDs have been prepared with high fluorescence quantum yield (FLQY: 44.8%). As-prepared NGCDs exhibit excellent photostability under continuous UV irradiation. Owing to the strong interaction between Ga3+ and the oxygen functional groups (e.g., hydroxyl, carboxyl), the fluorescent NGCDs chemosensor shows a highly sensitive and selective response (a detection limit of 209 nM) to Ga3+ in a wide concentration range of 0–20 μM. Further fluorescence lifetime analyses suggest the quenching mechanism appears to be a dynamic process.
Introduction
Gallium as a trace element is widely applied in electronic devices including sensors,1,2 solar cells3,4 and LEDs.5,6 As gallium consumption rises, the level of gallium in the environment is gradually increasing.7,8 Because large amounts of gallium compound waste are considered to be health-hazardous and carcinogenic, it is critically important to develop reliable methods for gallium ion (Ga3+) detection and gallium level monitoring in natural water.9,10 Up to now, some methods, such as ion exchange, adsorption, extraction, and atomic absorption spectrometry, have been developed for Ga3+ determination.11–14 These methods show good detection limits but are complex processes with high cost.15,16 Therefore, the development of one simple and cheap detection method for Ga3+ is still requisite.Carbon dots (CDs), as an emerging optical material, have provided a diverse range of prospective applications in bioimaging, sensors, photocatalysis and photovoltaic devices.17–25 Most recently, CDs have also been tentatively used as fluorescent chemosensors for metal ions detection including Hg2+, Fe3+, Cu2+, Al3+, Ag+.26–31 Indeed these high-quality CDs show excellent potency for metal ions detection. However, to our best knowledge, there is no any report about the CDs' chemosensor for Ga3+ detection. As a matter of fact, in the face of increasing Ga3+ pollution, it is the moment of truth to attempt to find a new kind of CDs as a chemosensor for Ga3+ detection.
Herein we explore a fluorescent nitrogen-doped graphitic carbon dots (NGCDs) chemosensor for label-free Ga3+ detection for the first time. By direct pyrolysis of ammonium citrate (AC) in air, highly fluorescent NGCDs (FLQY: 44.8%) are obtained. The as-synthesized NGCDs exhibit excellent photostability under continuous UV irradiation. The fluorescence of NGCDs can be quenched by Ga3+ ions, which may be attributed to the complexation between oxygen functional groups and Ga3+. The fluorescent NGCDs chemosensor shows a highly sensitive and selective response (a detection limit of 209 nM) to Ga3+ in a wide concentration range of 0–20 μM. Moreover, the fluorescence lifetime analyses suggest the quenching mechanism appears to be a dynamic process.
Experimental
Chemicals
All reagents and chemicals were of analytical grade and purchased from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). They were all used without further purification. Deionized water with a resistivity of 18.2 MΩ cm was used for all experiments.Synthesis of NGCDs
The NGCDs were synthesized by a one-pot pyrolysis of ammonium citrate (AC) directly. In a typical synthesis procedure, 1 g AC was put in a 10 mL beaker and heated to 200 °C moderately using a heating mantle. After 5 min, the AC changed to liquid. Then a uniform brown liquid was obtained about 10 min later, which means the formation of NGCDs. Dissolved in 50 mL water, the NGCDs were collected via dialysis for 2 days in super-purified water (3000 Da) for further characterization and use.Metal ion detection
In the selectivity experiment, Ga(NO3)3, AgNO3, NaCl, CdCl2, CoCl2, CuCl2, CsCl2, FeCl3, BiCl2, MnCl2, Ni(NO3)2, PbCl2, SbCl3, SnCl2, BaCl2, Zn(NO3)2 have been used as various ion sources. NGCDs solution (0.25 mgL−1, 100 μL) was mixed with the solution containing a fixed concentration of ions (5 mM). For the sensitive detection of Ga3+, NGCDs solution (0.25 mgL−1, 100 μL) was mixed with aqueous solution with different concentration of Ga3+. The PL spectra were recorded after reaction for a few minutes and the excitation wavelength was fixed at 350 nm for all the PL spectra.Characterization
The morphology of the N-doped GCDs is investigated by transmission electron microscopy (TEM, FEI Tecnai G-20) and atomic force microscopy (AFM, Asylum Research MFP-3D-BIO). Fourier transform infrared (FT-IR) spectra are obtained on a Bruker VERTEX70 FT-IR spectrometer ranged from 4000 to 400 cm−1. The carbon structure of N-doped GCDs was studied by using Raman microscope (HORIBA Jobin Yvon, HR800) and X-ray diffractometer (XRD, D/MAX-2000 PC). X-ray photoelectron spectra (XPS) are acquired with a Japan Kratos Axis Ultra HAS spectrometer using a monochromatic Al Kα source. Ultraviolet-visible (UV-vis) absorption spectra of F-CNPs were obtained using Shimadzu UV-2450 spectrophotometer. All the photoluminescence (PL) spectra are recorded on a Fluoromax-4 spectrofluorometer (HORIBA Jobin Yvon Inc.) equipped with a 150 W of xenon lamp as the excitation source.Result and discussion
Herein, highly fluorescent NGCDs were prepared via pyrolyzing ammonium citrate at 200 °C. Shown in Fig. 1a, the as-prepared NGCDs appear to be well dispersed and near orbicular in morphology. The NGCDs' sizes are mainly distributed in the range of 4–8 nm, with an average value of 5.8 nm (Fig. 1c). The atomic force microscopy (AFM) image (Fig. 1b) shows the height of the NGCDs, which is mostly distributed in the range of 0.5–2 nm (Fig. 1d). The typical XRD pattern (Fig. S1†) of the NGCDs shows a wide (002) peak around 0.34 nm, which is similar to GCDs synthesized by other methods.24 It indicates that carbonizing AC would produce graphitic structure. As shown in Fig. 1e, the Raman spectrum of the NGCDs has two typical peaks at 1354 cm−1 (D band) and 1595 cm−1 (G band).32 The full scan spectrum (Fig. 1f) tells that carbon, nitrogen and oxygen co-exist at the surface of NGCDs. The corresponding C1s, N1s, O1s peaks are located at ca. 285, 401, 532 eV, respectively. High-resolution XPS spectra (Fig. S2a–c†) and Fourier transform infrared (FTIR) spectrum (Fig. S3†) further confirm the presence of O–H, N–H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, COO–, C–N and C–OH. As shown in Table S1,† 9.2% of nitrogen and 61.6% of carbon also illuminate that NGCDs are nitrogen-doped.
O, COO–, C–N and C–OH. As shown in Table S1,† 9.2% of nitrogen and 61.6% of carbon also illuminate that NGCDs are nitrogen-doped.
To further study the optical properties of NGCDs, UV-vis absorption and PL characterization were carried out. As shown in Fig. 2a, the blue-fluorescent NGCDs exhibit two absorption peaks at ca. 235 nm and 337 nm, which correspond to π–π* transition of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond and n–π* transition of the C
C bond and n–π* transition of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond, respectively.33 The NGCDs show bright blue fluorescence having the maximum excitation wavelength and the maximum emission wavelength at 350 nm and 437 nm, respectively. Fig. 2b clearly exhibits the NGCDs have the maximum emission when excited at 350 nm while the NGCDs' emission intensity varies under the different excitations. The NGCDs' fluorescence quantum yield (FLQY) was calculated to be 44.8% with quinine sulfate (FLQY: 54%) as the reference (Fig. S4†), which is higher in comparison with the similar GCDs' materials.34 The fluorescence intensity of NGCDs exhibits a decrease with the temperature increasing from room temperature (RT) to 80 °C (Fig. S5†), which can be recovered when it is cooled down to RT. In addition, the fluorescence response of NGCDs at different pH value was also investigated. The stability test of NGCDs is involved in the Fig. 2c. The PL intensity of NGCDs aqueous solution decreases less than 10% after the UV irradiation for 1 h. As shown in Fig. 2d, it is interesting that the NGCDs' PL intensity linearly decreased with the pH decreasing in the acidic condition. That is to say, the carboxyl groups might be protonated on the surface of NGCDs in the strong acidic solutions. As a result, the fluorescence of NGCDs will be quenched due to the aggregation arising from strong protonation.25 It is also indirectly demonstrated that the PL intensity of NGCDs changed little under alkaline conditions. The above characterizations suggest the NGCDs possess excellent PL properties. These offer an advantage platform for the potential application in the field of the chemosensors.
O bond, respectively.33 The NGCDs show bright blue fluorescence having the maximum excitation wavelength and the maximum emission wavelength at 350 nm and 437 nm, respectively. Fig. 2b clearly exhibits the NGCDs have the maximum emission when excited at 350 nm while the NGCDs' emission intensity varies under the different excitations. The NGCDs' fluorescence quantum yield (FLQY) was calculated to be 44.8% with quinine sulfate (FLQY: 54%) as the reference (Fig. S4†), which is higher in comparison with the similar GCDs' materials.34 The fluorescence intensity of NGCDs exhibits a decrease with the temperature increasing from room temperature (RT) to 80 °C (Fig. S5†), which can be recovered when it is cooled down to RT. In addition, the fluorescence response of NGCDs at different pH value was also investigated. The stability test of NGCDs is involved in the Fig. 2c. The PL intensity of NGCDs aqueous solution decreases less than 10% after the UV irradiation for 1 h. As shown in Fig. 2d, it is interesting that the NGCDs' PL intensity linearly decreased with the pH decreasing in the acidic condition. That is to say, the carboxyl groups might be protonated on the surface of NGCDs in the strong acidic solutions. As a result, the fluorescence of NGCDs will be quenched due to the aggregation arising from strong protonation.25 It is also indirectly demonstrated that the PL intensity of NGCDs changed little under alkaline conditions. The above characterizations suggest the NGCDs possess excellent PL properties. These offer an advantage platform for the potential application in the field of the chemosensors.
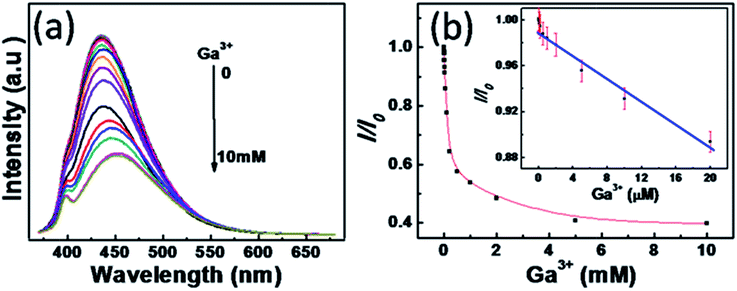
As known, metal ions can interact with GCDs by chelating with the oxygen functional groups of the surface and the π system of the graphitic structure.35 The chemical reaction stimulates the possible NGCDs-based chemosensor for metal ions detection. Herein, as-synthesized NGCDs have been attempted to be a fluorescent chemosensor for efficient Ga3+ detection. As shown in Fig. 3a, the fluorescence variation of NGCDs in the presence of different amounts of Ga3+ was studied to confirm the feasibility of Ga3+ detection. With the Ga3+ concentration increasing from 0 to 10 mM, the PL intensity gradually decreased to ∼40% from the initial value. Fig. 3b shows the dependence of I/I0 on the concentration of Ga3+, where I and I0 stand for the maximal PL intensity excited at the wavelength of 350 nm in the presence and absence of Ga3+, respectively. The PL intensity of NGCDs versus the concentrations of Ga3+ has a good linear relation with a correlation coefficient of 0.972 in the concentration range of 0–20 μM. Under the current experimental conditions, the detection limit of Ga3+ is estimated to be 209 nM on the basis of 3Sb/k (Sb refers to the standard deviation of the corrected blank signals of the NGCDs and k is the slope of the calibration curve). In addition to the high sensitivity for Ga3+, the selectivity of NGCDs towards different metal ions is also taken into account. Fig. 4 shows the various fluorescence intensity ratios of NGCDs in the absence and presence (5 mM) of different metal ions. In comparison with the ions (such as Ag+, Na+, Cd2+, Co2+, Cu2+, Cs2+, Fe3+, Bi2+, Mn2+, Ni2+, Pb2+, Sb3+, Sn2+, Ba2+, Zn2+) existing in the polluted environment, Ga3+ is much more sensitive to the NGCDs. Furthermore, we have not added any label reagent to improve the sensitivity and selectivity of NGCDs for Ga3+. Therefore, the highly fluorescent NGCDs are one good candidate of the label-free chemosensors for Ga3+ detection.
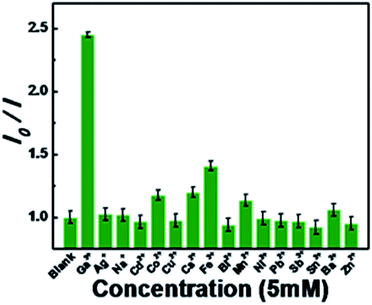 | ||
| Fig. 4 Fluorescence intensity ratio (I0/I) of the NGCDs solution without/with various individual metal ions, I0 and I are the emission intensities excited at 350 nm wavelength. | ||
To clear the fluorescence quenching mechanism, we experimented the fluorescence decays of NGCDs by time-correlated single-photon counting (TCSPC) without/with Ga3+ (Fig. 5a). The NGCDs lifetime (τ0) without Ga3+ is calculated to be 9.82 ns. After introducing 10 μM Ga3+, the NGCDs lifetime (τ) decreases to 9.57 ns. Furthermore, the τ comes to 7.97 ns and 7.1 ns while the Ga3+ concentrations are 100 μM and 5 mM, respectively. According to the Stern–Volmer equation, the fluorescence quenching should originate from collisional deactivation (dynamic quenching process).36 To further confirm it, ethylene diamine tetraacetic acid (EDTA, a competitive chelator) is added to Ga3+-quenched NGCDs solution. As shown in Fig. 5b, the fluorescence intensity of the Ga3+-quenched NGCDs solution cannot be recovered by EDTA. This phenomenon suggests the quenching process by Ga3+ is dynamic quenching.28,37 To find the real insight of the fluorescence quenching mechanism, more work still need to go.
 | ||
| Fig. 5 (a) Fluorescence decay traces of NGCDs by TCSPC in the different concentration of Ga3+. (b) Fluorescence spectra of NGCDs without/with Ga3+ and EDTA. | ||
Conclusions
In conclusion, the highly fluorescent NGCDs have been prepared via pyrolysis of ammonium citrate in air. Following the specific PL analyses, a new fluorescent label-free chemosensor of the NGCDs have been applied for highly sensitive and selective detection of Ga3+ in aqueous solution. The NGCDs based chemosensor has a detection limit of 209 nM for the Ga3+ in the concentration range of 0–20 μM. The synthesized NGCDs not only provide a simple and practical method for Ga3+ detection, but also expand a potential application range of other metal ions' chemosensor.Acknowledgements
We gratefully acknowledge the support from "973 Program" Special Funds for the Chief Young Scientist (2015CB358600), the Excellent Young Scholar Fund from National Natural Science Foundation of China (21422103), the Jiangsu Fund for Distinguished Young Scientist (BK20140010), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), and Jiangsu Scientific and Technological Innovation Team (2013). H. Luo thanks funding support from New Mexico EPSCoR with NSF-1301346.Notes and references
- J. M. Kikkawa and D. D. Awschalom, Nature, 1999, 397, 139–141 CrossRef CAS PubMed.
- H. Li, Y. Yang and J. Liu, Appl. Phys. Lett., 2002, 101, 073511 CrossRef PubMed.
- H. M. Kim, Y. H. Cho, H. Lee, S. I. Kim, S. R. Ryu, D. Y. Kim, T. W. Kang and K. S. Chung, Nano Lett., 2004, 4, 1059–1062 CrossRef CAS.
- R. Trotta, P. Atkinson, J. D. Plumhof, E. Zallo, R. O. Rezaev, S. Kumar, S. Baunack, J. R. Schröter, A. Rastelli and O. G. Schmidt, Adv. Mater., 2012, 24, 2668–2672 CrossRef CAS PubMed.
- J. Yoon, S. Jo, I. S. Chun, I. Jung, H. S. Kim, M. Meitl, E. Menard, X. Li, J. J. Coleman, U. Paik and J. A. Rogers, Nature, 2010, 465, 329–333 CrossRef CAS PubMed.
- M. Yao, N. Huang, S. Cong, C. Y. Chi, M. A. Seyedi, Y. T. Lin, Y. Cao, M. L. Povinelli, P. D. Dapkus and C. Zhou, Nano Lett., 2014, 14, 3293–3303 CrossRef CAS PubMed.
- A. V. Naumov, Russ. J. Non-Ferr. Met., 2014, 55, 270–276 CrossRef.
- H. Tavallal, P. Vahdati and E. Shaabanpur, Sens. Actuators, B, 2011, 159, 154–158 CrossRef PubMed.
- C. S. Ivanoff, A. E. Ivanoff and T. L. Hottel, Food Chem. Toxicol., 2012, 50, 212–215 CrossRef CAS PubMed.
- C. R. Chitambar, Int. J. Environ. Res. Public Health, 2010, 7, 2337–2361 CrossRef CAS PubMed.
- X. Shan, W. Wang and B. Wen, J. Anal. At. Spectrom., 1992, 7, 761–764 RSC.
- N. Hirayama, Y. Horita, S. Oshima, K. Kubono, H. Kokusen and T. Honjo, Talanta, 2001, 54, 857–862 CrossRef.
- B. Gong, X. Li, F. Wang and X. Chang, Talanta, 2000, 52, 217–223 CrossRef CAS.
- K. Junko, Y. Hiroshi, O. Hayato, Y. Takehiko and F. Takeshi, Anal. Chim. Acta, 2009, 635, 207–213 CrossRef PubMed.
- J. Y. Noh, S. Kim, I. H. Hwang, G. Y. Lee, J. Kang and S. H. Kim, Dyes Pigm., 2013, 99, 1016–1021 CrossRef CAS PubMed.
- M. Grabarczyk and C. Wardak, J. Environ. Sci. Health, Part A: Toxic/Hazard. Subst. Environ. Eng., 2014, 49, 1142–1148 CrossRef CAS PubMed.
- S. N. Baker and G. A. Baker, Angew. Chem., 2010, 49, 6726–6744 CrossRef CAS PubMed.
- Q. Liu, B. Guo, Z. Rao, B. Zhang and J. R. Gong, Nano Lett., 2013, 13, 2436–2441 CrossRef CAS PubMed.
- H. T. Li, Z. H. Kang, Y. Liu and S. T. Lee, J. Mater. Chem., 2012, 22, 24230–24253 RSC.
- M. M. Yang, H. Li, J. Liu, W. Q. Kong, S. Y. Zhao, C. X. Li, H. Huang, Y. Liu and Z. H. Kang, J. Mater. Chem. B, 2014, 2, 7964–7970 RSC.
- S. Zhu, Q. Meng, L. Wang, J. Zhang, Y. Song, H. Jin, K. Zhang, H. Sun, H. Wang and B. Yang, Angew. Chem., 2013, 125, 4045–4049 CrossRef.
- Z. Lin, W. Xue, H. Chen and J. Lin, Anal. Chem., 2011, 83, 8245–8251 CrossRef CAS PubMed.
- Q. Li, S. Zhang, L. Dai and L. Li, J. Am. Chem. Soc., 2012, 134, 18932–18935 CrossRef CAS PubMed.
- Y. Li, Y. Hu, Y. Zhao, G. Shi, L. Deng, Y. Hou and L. Qu, Adv. Mater., 2011, 23, 776–780 CrossRef CAS PubMed.
- V. Gupta, N. Chaudhary, R. Srivastava, G. D. Sharma, R. Bhardwaj and S. Chand, J. Am. Chem. Soc., 2011, 133, 9960–9963 CrossRef CAS PubMed.
- Y. Guo, Z. Wang, H. Shao and X. Jiang, Carbon, 2013, 52, 583–589 CrossRef CAS PubMed.
- Y. L. Zhang, L. Wang, H. C. Zhang, Y. Liu, H. Y. Wang, Z. H. Kang and S. T. Lee, RSC Adv., 2013, 3, 3733–3738 RSC.
- F. Wang, Z. Gu, W. Lei, W. Wang, X. Xia and Q. Hao, Sens. Actuators, B, 2014, 190, 516–522 CrossRef CAS PubMed.
- Q. Qu, A. W. Zhu, X. L. Shao, G. Y. Shi and Y. Tian, Chem. Commun., 2012, 48, 5473–5475 RSC.
- Z. Fan, Y. Li, X. LI, L. Fan, S. Zhou, D. Fang and S. Yang, Carbon, 2014, 70, 149–156 CrossRef CAS PubMed.
- Z. Qian, J. Ma, X. Shan, H. Feng, L. Shao and J. Chen, Chem.–Eur. J., 2014, 20, 2254–2263 CrossRef CAS PubMed.
- A. C. Ferrari, J. C. Meyer, V. Scardaci, C. Casiraghi, M. Lazzeri, F. Mauri, S. Piscanec, D. Jiang, K. S. Novoselov, S. Roth and A. K. Geim, Phys. Rev. Lett., 2006, 97, 187401 CrossRef CAS.
- X. Zhang, Y. Zhang, Y. Wang, S. Kalytchuk, S. V. Kershaw, Y. Wang, P. Wang, T. Zhang, Y. Zhao, H. Zhang, T. Cui, Y. Wang, J. Zhao, W. W. Yu and A. L. Rogach, ACS Nano, 2013, 7, 11234–11241 CrossRef CAS PubMed.
- Z. Yang, M. Xu, Y. Liu, F. He, F. Gao, Y. Su, H. Wei and Y. Zhang, Nanoscale, 2014, 6, 1890–1895 RSC.
- S. Park, K. S. Lee, G. Bozoklu, W. W. Cai, S. T. Nguyen and R. S. Ruoff, ACS Nano, 2008, 2, 572–578 CrossRef CAS PubMed.
- L. J. Fan, Y. Zhang, C. B. Murphy, S. E. Angell, M. F. L. Parker, B. R. Flynn and W. E. Jones Jr, Coord. Chem. Rev., 2009, 253, 410–422 CrossRef CAS PubMed.
- H. Huang, L. Liao, X. Xu, M. Zou, F. Liu and N. Li, Talanta, 2013, 117, 152–157 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra15431b |
| This journal is © The Royal Society of Chemistry 2015 |