Isoindoline-derived ligands and applications
Róbert Csonka,
Gábor Speier and
József Kaizer*
Department of Chemistry, University of Pannonia, 8201 Veszprém, Hungary. E-mail: kaizer@almos.vein.hu
First published on 4th February 2015
Abstract
During the past decade isoindoline-based ligands became the subject of growing interest due to their modular set-up. In this review the structure and reactivity of these ligands and their transition metal complexes are covered. Beyond the discussion of the structural properties particular attention is paid to the expanding fields of applications of these compounds.
1. Introduction
The first tridentate bis(arylimino)isoindoline type ligand with a N,N,N binding mode was introduced in the early 1950s by Elvidge and Linstead.1,2 Although it was clear from the beginning that this compound family can act as potent metal chelators, their coordination chemistry started to make considerable progress from the late 1970s and 1980s.3–5 At this time the first X-ray structure was published6 and also alternatives for ligand synthesis.7 Concerning the possible starting materials diiminoisoindoline (DII or 2) and phthalocyanines, they have been utilized as building blocks of supramolecular architectures in their metal complex form,8 and optical properties have been researched and exploited as well.9 The tunability of the parent materials creates a wide variety of bi- and tridentate ligands. Some in vitro biological tests showed that by small modifications on the isoindole base antiproliferative activity can be achieved even without the presence of any metals,10 furthermore isoindoline carbamates have a structural key role in diuretic or blood pressure regulator agents.11,12 By the application of chiral sidearm enantioselective catalysis can be carried out13 that is important both in industrial and medicinal chemistry.The main structural features involve bis(arylimino), bis(alkylimino) and monosubstituted (asymmetric) isoindolines. Another structure of interest belongs to the phthalazine type molecules originated from bis(R-imino)isoindolines (BII or 3) by ring expansion with hydrazine hydrate.14 The transition metal complexes of these formulations have applications from enzyme mimics15–17 to catalytic hydrogenation18 or in photophysics,19 that is discussed in this review.
2. Synthetic routes, characteristics
2.1. In solution synthesis
The more than six decades long history of bis(R-imino)isoindolines (3) synthesis created two main methods that are generally followed by scientists (Scheme 1). The applicability of these methods was partly reviewed by Tamgho et al. recently.20 Fifteen old and new BII ligands, containing no other heteroatoms, were examined in their work and the original synthetic method1 is compared to the more recent Siegl's method.3 It was pointed out that the latter is a more suitable way of producing bulky and cyclic derivatives. According to the Linstead method phthalonitrile (1) has to be converted to 1,3-diiminoisoindoline (2) first (it is commercially available nowadays) then the imine condenses with amines in ethanol during a 24 hours reflux time. In Siegl's method phthalonitrile is directly used in a condensation reaction in the presence of CaCl2. The reaction time is considerably long (usually 48 hours) but yields are higher.2.2. Fusion synthesis
The least used method at the synthesis of 3 is the fusion reaction of phthalonitrile 1 with primary amines at high temperature (180–190 °C). Seemingly, it is a modification of the Siegl method but there are considerable advantages, namely it requires no solvent and reaction time is shorter (average 18 hours), while yields are above 80%.21,22 The expected yield of the previous methods is 50–80%.2.3. Structure based classification
A nice variety of derivatives have been created from 1 and 2. The major groups of these compounds can be named based on the structure: aliphatic BII (aliBII),20 aromatic BII (aroBII),1 unsymmetrical BII (unsyBII),23 chiral BII (chiBII)13 and aminophthalazines (PAPs)14 (Fig. 1). Phthalocyanines or subporphyrines are the cyclic formulations of DII but their synthesis requires different strategy from the previously mentioned molecules, furthermore their applications and research are both well documented.24,25By the presence of aliphatic moieties on BII the chelating capacity is usually limited. The lack of heteroatoms favors the mono-, or bidentate ligand formation that is based on the coordination to the endocyclic NH and one imino side arm on the isoindole ring. Although, aliBIIs have been reported since the first appearance of BIIs,2,20,26–31,54 complexes of this kind have been synthesized only once by Maleev et al. In this work, the coordination of bidentate aliBII to rare earth metals is discussed. They are observed both in terminal and bridge positions using all three coordination sites in the latter case.32
The less common monodentate behavior of BII has been detected by our group33 by blocking the N atoms of 1,3-bis(2′-pyridylimino)isoindoline – BPI (7). The zwitterionic copper(I) compound showed moderate catechol oxidase activity.
BPI and its substituted derivatives are the most researched aroBIIs. They are considered pincer type ligands based on the tridentate coordination mode and the aromatic planarity around the metal ions. They are monoanionic, nevertheless complexes of protonated ligands can form as well with suitable metal ions and the proper conditions applied.34 The meridional configuration is open enough to host other coligands or various substrates. A second identical ligand stabilizes the structure creating an octahedral, homoleptic complex, although the active species remains usually the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ligand to metal ratio form.
1 ligand to metal ratio form.
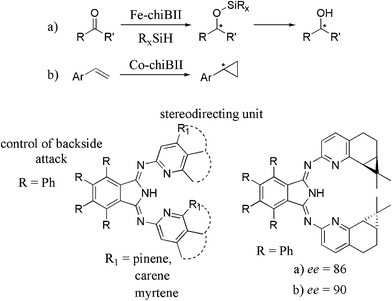
Because of the favorable characteristics of BPI chiral side chains can be attached as well. Fletcher et al. reported a facile one pot synthesis of chiral aminopyridines.35 To obtain any optically active BII is only one step away. Myrtene, α-pinene and carene have been used to build chiBIIs. Concerning enantioselective catalysis only a few number of work can be found done by Bröring and Gade.13,36–38
The preparation of BIIs by all known methods is a two-step procedure. The partial condensation of the starting material might happen if reaction time is not adequate. This can be used to our advantage if the goal is to achieve unsyBII. Since intramolecular C–H bond activation has been observed multiple times by Pd complexes,39–41 a better understanding on the mechanism is greatly expected. In order to study this phenomenon several unsyBIIs have been synthesized.42,43 Unsymmetry have been proved to be useful in the research of catecholase activity as well.23
Versatility of BPI goes beyond its applications and side chain variations. Simply, by the addition of hydrazine hydrate a ring expansion occurs on the isoindoline core that reveals an entirely new chemistry (Scheme 2).14
PAP (4) has a similar behavior to 3. It is anionic and it can form any metal complexes both in protonated or deprotonated state. Acetato, hydroxo, oxo, chloro etc. bridges are often observed in the binuclear center. The number of coordinated anions represented by L on Scheme 2 can vary depending on the metal salt used.
In Fig. 2 ligands have been collected that are the derivatives of both 3 and 4 showing only the R-group and their reference number used in this review.
2.4. Characterization
The two imino moieties on the isoindole core have a strong impact on the planar structure of both BII and its coordination complexes. The double bond between N and C atoms extends aromaticity and increases the rigidity of the system. It can easily be detected by FT-IR and a characteristic NMR signs can be attributed to one of these arms when mostly aliphatic residues are attached to the BII.20The second key structural element is the endocyclic NH group. It is responsible for an anionic character observed with metal ions. Depending on the electronic strength of R-groups, the anion applied with the metal ion, the solvent used, the inert atmosphere and the presence of bases both deprotonated and protonated complexes can be formed.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N vibrations modes that are mostly influenced by this proton appear as two very strong bands in the 1600 cm−1 to 1660 cm−1 interval in the free ligands. These bands are suppressed if coordination happens by deprotonation. In this case they shift below 1600 cm−1 loosing intensity at the same time. In case of nondeprotonated complexation the strong vibrations above 1600 cm−1 remain visible and additionally other intense bands appear around 1550 cm−1.34,46,74
N vibrations modes that are mostly influenced by this proton appear as two very strong bands in the 1600 cm−1 to 1660 cm−1 interval in the free ligands. These bands are suppressed if coordination happens by deprotonation. In this case they shift below 1600 cm−1 loosing intensity at the same time. In case of nondeprotonated complexation the strong vibrations above 1600 cm−1 remain visible and additionally other intense bands appear around 1550 cm−1.34,46,74The electronic absorption spectrum of the ligands 3 show a multiple band pattern. The π–π* transitions are created by the extended π-bond system and they appear in the 360–500 nm region. Upon complex formation small (2–20 nm) red or blue shifts are observed. Lower energy bands are contributed to charge transfer transitions from metal ion to the ligand. A selection of absorption bands of various BII and their homoleptic transition metal complexes has been collected in Table 1.
| Ligand | π–π* CT bands of the free ligands λmax/nm (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) ε) |
[M(L)2]λmax/nm (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) in DMF, (metal complex) ε) in DMF, (metal complex) |
Ref. |
|---|---|---|---|
| a Ligand synthesis.b Complex synthesis and X-ray.c Spectroscopic data. | |||
| 7 | 410 (3.99), 386 (4.23), 366 (4.19) in DMF | 440 (4.58), 410 (4.81), 390 (4.75) (FeII) | 1a, 48b and 22c |
| 8 | 408 (4.11), 385 (4.29), 366 (4.24) in DMF | 450 (4.65), 423 (4.73), 332 (4.45), 306 (4.53) (MnII) | 1a, 49b, 22c and 49c |
| 9 | 470 (4.01), 440 (4.30), 413 (4.31), 388 (4.35), 369 (4.37) in DMF | 493 (4.28), 453 (4.49), 389 (4.40), 367 (4.45) (FeII) | 50a, 22b,c and 51b,c |
| 474 (4.71), 440 (4.86), 414 (4.86) 364 (4.94), 287 (4.87) (FeIII) | |||
| 10 | 485 (3.18), 448 (3.86), 420 (4.05), 396 (4.01), 374 (3.94) in DMF | 476 (4.11), 433 (4.54), 410 (4.55), 333 (4.38), 319 (4.40), 286 (4.45) (CoII) | 52a, 53b, 22c and 53c |
3. Transition metal complexes of BII ligands
Structural and spectroscopic features of BII have been collected in details up to this point. The second part of this review will focus on the metal complexes, their unique characteristics and possible applications. Chemical industry requires these days the endless development of chiral catalyst, photoactive compounds, or mild CH and CC bond activating agents just to mention the hottest topics. In pharmaceutical industry green chemistry is the standard. In order to copy metalloenzymes and to find a better way to mimic their reactions there is a continuous challenge for science to create new, efficient complexes.3.1. Complexes of 3B to 6B metals
Vanadium, chromium and molybdenum are typical candidates for complex formation from group B elements. Interestingly, only two papers have been reported with BII ligands from the same author Baird et al. up to this date.55,56 Dimolybdenum was chosen to synthesize planar molecules with BPI (7) and 4′,6′-Me2BPI (35) ligands. One of the complexes was successfully characterized by X-ray revealing an unusual coordination mode (Fig. 3). | ||
| Fig. 3 A dimolybdenum complex (36) with BPI (7).56 | ||
As a remarkable feature of 36, one molybdenum ion is coordinated to an imino nitrogen rotating a pyridyl arm into a position where binding to the dimetal unit is not possible. Similar “imino binding” has been documented only once with an aliBII ligand32 [1,3-bis(isopropylimino)isoindolinate] but it could be expected in that case on the account of missing coordinating sidearms.
3.2. Complexes of 7B metals
Among the biogenic elements manganese has a distinctive role. A large variety of enzymes bear manganese(II or III) cofactor. Most of them participate in the defense mechanism against superoxide free radicals such as superoxide dismutase (SOD)57 and catalase enzymes.58 All those BII complexes that have been created contain only this element from group 7B (Table 2).Table 2 contains the characteristic data of the manganese complexes of BIIs. The complexes are identified using the abbreviations reported in the original manuscripts with the corresponding R-group from Fig. 2, being denoted for reference with this review. Tau values are valid only for five coordinate structures. The numbers show a nearly perfect trigonal bipyramid (42) and an average, moderately distorted TBPY (38). The influential factors such as oxidative atmosphere, bases and the right anion that greatly determine the BII structure will be described in the section of Fe-group elements due to the greater amount of research data. The distance between the amino group of the isoindoline core and the metal ion varies in a narrow interval among the manganese complexes. The difference is prominent at +2 (45) and +3 (46) oxidation states when the same ligand coordinates that can be explained by Jahn–Teller effect. Fig. 4 also emphasizes this difference. Only the Mn2+ complexes fit to a linear tendency with the increasing Nind–Mn2+ distance.
| Ligand | Complex | NPy–Mna (Å) | Nind–Mnb (Å) | τ | Ref. |
|---|---|---|---|---|---|
| a The average pyridyl N–Mn distance.b The isoindoline N(H)–Mn distance. | |||||
| 7 | [MnII(ind)2] (37) | 2.295 | 2.163, 2.143 | — | 49 |
| 7 | [MnII(indH)Cl2] (38) | 2.249 | 2.153 | 0.69 | 17 |
| 11 | [MnII(3′Me-BPI)2] (39) | 2.293 | 2.144, 2.151 | — | 21 |
| 8 | [MnII(4′Me-ind)2] (40) | — | — | — | 49 |
| 12 | [MnII(6′Me2indH)(H2O)2(CH3CN)](ClO4)2 (41) | — | — | — | 16 |
| 9 | [MnII(bimindH)Cl2](DMF) (42) | 1.959 | 2.007 | 0.93 | 50 |
| 9 | [MnII(bimind)2] (43) | — | — | — | 15 |
| 13 | [MnII(Mebimind)2] (44) | — | — | — | 15 |
| 10 | [MnII(BTI)2] (45) | 2.220 | 2.211, 2.220 | — | 15 |
| 10 | [MnIII(BTI)2] (46) | 2.146 | 1.968, 1.967 | — | 59 |
3.3. Complexes of 8B metals
Iron, cobalt and nickel complexes of BIIs are reported in the greatest number. Compared to manganese, iron also has the affinity to form a broad scale of oxygen adducts. Hereafter, less surprisingly Fe(R′-BPI)x (x = 1,2) are likely to be applied and tested as enzyme models.22,34,62,64,66Cobalt and nickel complexes of the title ligands are fairly rare although they have been used multiple times in the oxidation, hydroxylation of hydrocarbons (see also chapter 4.2.).60,61 The “pincer” shape is also an optimal platform for enantioselective catalytic reactions.36 Interaction with DNA have been studied in order to develop DNA cleaving therapeutic agents and non-radioactive probes for DNA through Co(BPI)s.62
| Ligand | Complex | NPy–Fea (Å) | Nind–Feb (Å) | τ (Φ) | Ref. |
|---|---|---|---|---|---|
| a The average pyridyl N–Fe distance.b The isoindoline N(H)–Fe distance. | |||||
| 7 | [FeII(ind)2] (47) | 2.240 | 2.069 | — | 48 |
| 7 | [FeII(ind)(CH3CN)3](ClO4)2 (48) | 2.200 | 2.072 | — | 34 |
| 7 | [FeIII(μ-O)(ind)2Cl2]THF (49) | 2.153 | 1.998 | 0.88 | 63 |
| 7 | [FeIII(ind)Cl2] (50) | 2.148 | 1.963 | 0.86 | 64 |
| 8 | [FeIII(4′Me-ind)Cl2] (51) | 2.144 | 1.978 | 0.77 | 65 |
| 9 | [FeIII(bimind)2] (52) | 1.979 | 1.912, 1.928 | — | 51 |
| 9 | [FeII(bimind)Cl2] (53) | 2.067 | 2.045 | — | 66 |
| 13 | [FeII(Mebimind)2] (54) | 2.136 | 2.057, 2.053 | — | 22 |
| 10 | [FeIII(BTI)2]MeCN (55) | 2.002 | 1.946, 1.952 | — | 22 |
| 10 | [FeIII(BTI)2] (56) | 1.982 | 1.928, 1.922 | — | 59 |
| 10 | [FeIII(BTI)Cl2] (57) | 2.095 | 2.019 | 0.83 | 65 |
| 14 | [FeIII(5′Me-BTI)Cl2] (58) | 2.098 | 2.029 | 0.59 | 67 |
| 15 | [FeIII(μ-O)(benzBTI)2Cl2] (59) | 2.165 | 2.064, 2.052 | 0.76 | 68 |
| 16 | [FeIICI(DMSO)(thqbpi)] (60) | 2.169 | 1.996 | 0.31 | 69 |
| 16 | [FeII(thqbpi)(CH2SiMe3)] (61) | 2.194 | 2.002 | 0.65 | 70 |
| 7 | [FeII(4-MeBPI)2] (62) | 2.085 | 1.979 | — | 61 |
A large number of transition metal complexes of BIIs have been reported by Robinson et al. in 1967.71 Unfortunately, at this time the lack of accurate analytical techniques limited the clear insight into fine structures. Nowadays, it is known that anions may participate in the inner and outer coordination sphere of the crystals. Similar roles have been assigned to solvent molecules too. The presence of water or non-inert atmosphere increases the chance of oxo-bridged formulations or the replacement of either anions or other solvents in the first coordination sphere (or inner sphere). Based on the available structures and synthetic procedures some conclusions can be made on complex formation. The iron(II)perchlorate and acetate salts tend to result in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (ligand–metal) complex (52, 54, 62, furthermore 37, 39 with manganese content) mainly with a help of some base (e.g. triethylamine). In the absence of any base and under inert atmosphere protonated 1
1 (ligand–metal) complex (52, 54, 62, furthermore 37, 39 with manganese content) mainly with a help of some base (e.g. triethylamine). In the absence of any base and under inert atmosphere protonated 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex can be achieved in a good coordinating solvent such as acetonitrile (48). Chlorides seem to favor the TBPY-5 set. There are only 1
1 complex can be achieved in a good coordinating solvent such as acetonitrile (48). Chlorides seem to favor the TBPY-5 set. There are only 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes reported both with iron(II) and iron(III)chlorides (50, 51, 58, similarly with Mn: 38, 42). Two precedents show that a μ-oxo bridge forms between two iron cores with FeCl3 (49, 59) under air.
1 complexes reported both with iron(II) and iron(III)chlorides (50, 51, 58, similarly with Mn: 38, 42). Two precedents show that a μ-oxo bridge forms between two iron cores with FeCl3 (49, 59) under air.
In spite of the attempts to obtain FeII(BTI)2 two independent work proved the viability of FeIII(BTI)2 (55, 56) submitted almost at the same time.22,59 They are seemingly identical (if anions are ignored) but X-ray structures reveal more. If average Naromatic–Fe distances are plotted against NInd–Fe distances high- and low-spin complexes can be separated (Fig. 5). There is Mössbauer spectra available to support the high-spin nature of 47 and 54.22,48 In a work by Pap et al. a low-spin Fe3+ complex (52) has been synthesized from its high-spin Fe2+ counterpart.51 55 was obtained from the Fe2+ form as well but less harsh conditions were applied.22 At the same time 56 was prepared directly from a Fe3+ salt undoubtedly creating a high-spin complex.
The only ruthenium complexes of BII available in literature have been presented by Pitchumony et al.,72 Gagné et al.73,74 and Tseng et al.75 all together with three X-ray structures (63–65) (Fig. 6). Ligands 7, 8 and 12 (respectively) were involved in the investigations. The resolved structure in the first case (63) contains a ligand fracture (1,2-dicyanobenzene) impurity that has an unusual monodentate connection to the metal center. Ruthenium complexes of 8 have been synthesized in various forms, just as Ru(4′-MeLH)Cl3 and Ru(4′-MeL)2. They were identified by spectroscopic methods and electrochemical and catalytic oxidative properties were tested. Complexes of 12 have PPh3 coligands RuII(6′Me-BPI)(PPh3)Cl (64) and HRuII(6′Me-BPI) (PPh3)2 (65). They catalyze base-free and chemoselective alcohol dehydrogenation.
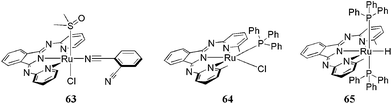 | ||
| Fig. 6 Representation of known ruthenium structures (63–65).72,75 | ||
Cobalt complexes of any kind favor the octahedral symmetry more than any other metals. The absence of the Werner-type arrangement is observed only in a couple of cases (72, 73, 75, 76, 78 – Table 4). 75–78 from these molecules have been intentionally converted to the TBPY-5 structure in order to investigate activity in radical polymerization.77 A rarely identified structure of 74 was achieved by pyridine displacement from the metal dialkyl precursor (py)2Co(CH2SiMe3)2.
| Ligand | Complex | NPy–Coa (Å) | Nind–Cob (Å) | τ (Φ) | Ref. |
|---|---|---|---|---|---|
| a The average pyridyl N–Co distance.b The isoindoline N(H)–Co distance. | |||||
| 7 | [CoIII(ind)2](ClO4)MeOH (66) | 1.985 | 1.889 | — | 62 |
| 7 | [CoIII(BPI)(OBz)(OO-t-Bu)] (67) | 1.955 | 1.845 | — | 60 |
| 9 | [CoII(bimind)OAc DMF] (68) | 2.093 | 2.040 | — | 66 |
| 10 | [CoII(BTI)2] (69) | 2.137 | 2.069, 2.073 | — | 53 |
| 10 | [CoIII(BTI)2] (70) | 1.959 | 1.913, 1.931 | — | 59 |
| 11 | [CoII(3′Me-ind)2] (71) | 2.196 | 2.004, 2.005 | — | 76 |
| 16 | [CoIICl(Me2SO)(thqbpi)] (72) | 2.161 | 1.941 | 0.51 | 69 |
| 16 | [Co2II(OAc)(thqbpi)2(MeOH)(EtOH)] (73) | 2.148 | 1.941, 1.939 | 0.54 | 69 |
| 17 | [CoII(CH2SiMe3)(pentbpi)] (74) | 2.076 | 1.949 | 0.67 | 37 |
| 18 | [Co2II(dihexyl1-tBu-bpi)2(OAc)] (75) | 2.126 | 1.983, 1.975 | 0.71 | 77 |
| 19 | [CoII(acac)(5′chloro-bpi)] (76) | 2.159 | 1.969 | 0.64 | 77 |
| 19 | [CoII(acac)(3,4-dichloro-5′chloro-bpi)(MeOH)] (77) | 2.177 | 2.021 | — | 77 |
| 19 | [CoII(acac)(3,4-dichloro-5′chloro-bpi)] (78) | 2.156 | 1.972 | 0.50 | 77 |
The two other members – rhodium and iridium – of this group belong to the rarest elements of the Earth's crust. A paper by Gade's research group presented a BII complex of the second densest element. IrI(18) (COD) with cyclooctadiene coordination was successfully identified by X-ray resulting in a OC-6 structure.78 18 behaves as a bidentate ligand that was observed only at lanthanides32 and at aliBIIs. A greater number of carbonyl complexes of the corresponding metals with aroBIIs were reported by Siegl.79 These compounds were identified by IR and elemental analysis and their reactivity toward alkyl and acyl halides was tested.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 square–planar complex. It complies with 83 that was synthesized from nickel(II)chloride and 80 that originates from another halogenated Ni complex (Table 5). Further acetato and chloro complexes have been prepared by Siegl7 from aminopyridine-BPI derivatives. The 2
1 square–planar complex. It complies with 83 that was synthesized from nickel(II)chloride and 80 that originates from another halogenated Ni complex (Table 5). Further acetato and chloro complexes have been prepared by Siegl7 from aminopyridine-BPI derivatives. The 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes (79, 81) could form with the help of deprotonating triethylamine.
1 complexes (79, 81) could form with the help of deprotonating triethylamine.
| Ligand | Complex | NPy–Nia (Å) | Nind–Nib (Å) | Φ | Ref. |
|---|---|---|---|---|---|
| a The average pyridyl N–Ni distance.b The isoindoline N(H)–Ni distance. | |||||
| 7 | [NiII(ind)2] (79) | 2.168 | 2.024 | — | 15 |
| 7 | [NiII(ind)(2-Clpcyd)] (80) | 1.965 | 1.827 | — | 80 |
| 8 | [NiII(4′Me-ind)2] (81) | 2.180 | 2.017 | — | 15 |
| 9 | [NiII(bimind)OAc DMF] (82) | 2.069 | 2.011 | — | 66 |
| 20 | [NiII(boxmi)Cl] (83) | 1.911 | 1.904 | 0.07 | 36 |
The enantioselective fluorination of oxindoles and β-ketoesters to the corresponding products is effectively catalyzed by 83 with high ee and noteworthy yield.36
The most redundant group of isoindoline complexes is undoubtedly the one with palladium core. Its popularity can be attributed to the cyclopalladation phenomena. Unlike with other transition metals, unique N,N,C type coordination can occur with BII that was documented by Dietrich et al.39 Steric hindrance on the pyridyl moiety and palladium excess are the influential factors of cyclometallation.40 The flexibility of BIIs allows a flip on the axis of one C–N bond that is attributed to an intramolecular strain.41 After C–H and C–C bond activation takes place further PdII can coordinate to exocyclic N atoms (87). Exploiting this remarkable feature of palladium S-coordination (114) was observed on one thiazole subunit of BTI ligand (26) that was unprecedented before (Fig. 7).86
 | ||
| Fig. 7 Exocyclic- (left) and S-coordination (right) of palladium complexes.41,86 | ||
All Pd complexes are planar or pseudoplanar, no other structures have been recorded. Unsymmetrical complexes were not included in Table 6. Kleeberg and Bröring reported a series of such complexes with unusual structural features.43 The new complexes had tBu-selenazol-pyridine and tBu-thiazole-pyridine sidearms on their ligands. In both of their packing pattern large voids were comprised that could be suitable even for small molecule storage according to the authors.
| Ligand | Complex | NPy–Pda (Å) | Nind–Pdb (Å) | Φ | Ref. |
|---|---|---|---|---|---|
| a The average pyridyl N–Pd distance.b The isoindoline N(H)–Pd distance. | |||||
| 7 | [PdII(BPI)(OAc)] (84) | 2.038 | 1.946 | 0.13 | 41 |
| 8 | [PdIICl(4-(HOCH2CH2)-10-MeBPI)] (85) | 2.063 | 1.965 | 0.09 | 81 |
| 12 | [PdII(6′Me-ind)(Cl)] THF (86) | 2.152 | 1.967 | 0.19 | 39 |
| 12 | [PdII4(6′Me-BPI)2(OAc)4] (87) | 2.174 | 1.987 | 0.16 | 41 |
| 18 | [PdIICl(4-Me-10-tBuBPI)] (88) | 2.065 | 1.957 | 0.18 | 18 |
| 21 | [PdIICl(11-Me-BPI)] (89) | 2.058 | 1.93, 1.94 | 0.09 | 18 |
| 22 | [PdIICl(11-Br-BPI)] (90) | 2.061 | 1.962 | 0.16 | 18 |
| 23 | [PdIICl(11-TMS-ethynyl-BPI)] (91) | 2.049 | 1.958 | 0.21 | 18 |
| 24 | [PdIICl(11-Ph3Si-ethynyl-BPI) (92) | 2.031 | 1.952 | 0.23 | 18 |
| 25 | [PdII(pinBPI)(OAc)] (93) | 2.053 | 1.936 | 0.04 | 38 |
| 25 | [PdIICl(pinBPI)] (94) | 2.053 | 1.962 | 0.20 | 38 |
| 26 | [PdII(4-MeBTI)(OC(NH2)Me)]+ BArF− (95) | 2.036 | 1.968 | 0.29 | 82 |
| 26 | [PdII(4-MeBTI)(dmf)]+ BArF− (96) | 2.026 | 1.968 | 0.24 | 82 |
| 26 | [PdII(4-MeBTI)(OCHO)]+ BArF− (97) | 2.034 | 1.976 | 0.17 | 82 |
| 26 | [PdII(4-MeBTI)(OC(NHPh)2)]+ BArF− (98) | 2.045 | 1.962 | 0.19 | 82 |
| 26 | [PdII(4-MeBTI)(SMe2)]+ BArF− (99) | 2.024 | 2.009 | 0.36 | 82 |
| 26 | [PdII(4-MeBTI)(SeMe2)]+ (100) | 2.020 | 2.010 | 0.36 | 82 |
| 26 | [PdII(4-MeBTI)(OAc)] (101) | 2.046 | 1.978 | 0.21 | 83 |
| 26 | [PdII(4-MeBTI)(Cl)] (102) | 2.038 | 1.998 | 0.33 | 83 |
| 26 | [PdII(4-MeBTI)(PMe3)] B(ArF)4 (103) | 2.018 | 2.036 | 0.44 | 84 |
| 26 | [PdII(4-MeBTI)(tBuNH2)] B(ArF)4 (104) | 2.030 | 1.988 | 0.34 | 84 |
| 26 | [PdII(4-MeBTI)(PhNH2)] B(ArF)4 (105) | 2.031 | 1.974 | 0.28 | 84 |
| 26 | [PdII(4-MeBTI)(Py)] B(ArF)4 (106) | 2.064 | 1.973 | 0.03 | 84 |
| 26 | [PdII(4-MeBTI)(2,6-Me2py)] B(ArF)4 (107) | 2.055 | 1.969 | 0.01 | 84 |
| 26 | [PdII(4-MeBTI)(MeCN)] B(ArF)4 (108) | 2.044 | 1.961 | 0.28 | 84 |
| 26 | [PdII(4-MeBTI)(MeNC)] B(ArF)4 (109) | 2.048 | 2.000 | 0.27 | 84 |
| 27 | [PdII(3,6-MepMeOi)(OAc)] (110) | 2.078 | 1.957 | 0.27 | 85 |
| 27 | [PdII(3,6-Me2-BPI)(OAc)] (111) | 2.082 | 1.949 | 0.27 | 41 |
| 28 | [PdII(iPrBTI)(OAc)] (112) | 2.027 | 1.989 | 0.21 | 85 |
| 29 | [PdII(6-Me-bpmi)(OAc)] (113) | 2.054 | 1.958 | 0.02 | 41 |
| 30 | [PdII(4-tBu-BTI)] (114) | 1.986 | 2.076 | 0.03 | 86 |
| 30 | [PdII(4-tBu-BTI)(Py)] (115) | 2.010 | 2.132 | 0.09 | 86 |
The small number of platinum complexes (Table 7) have similar structural motives to the ones in the previous chapter. Most of them was presented by Wen et al.19,87 They have square-planar geometry around the metal ion with chloride and aromatic coligands. Photophysical properties like luminescence are in the focus of recent research in both biologic (ion sensors) and industrial solutions (photocatalytic hydrogen production).
| Ligand | Complex | NPy–Pta (Å) | Nind–Ptb (Å) | Φ | Ref. |
|---|---|---|---|---|---|
| a The average pyridyl N–Pt distance.b The isoindoline N(H)–Pt distance. | |||||
| 7 | [PtII(BPI)pyridine](PF6) (116) | 2.058 | 1.964 | 0.15 | 87 |
| 7 | [PtII(BPI)(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CC6H5)] (117) CC6H5)] (117) |
2.046 | 1.985 | 0.10 | 19 |
| 7 | [PtII(BPI)(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CC6H4But-4] (118) CC6H4But-4] (118) |
2.050 | 1.995 | 0.13 | 19 |
| 7 | [PtII(HL3BPI)(Cl)] (119) | 2.039 | 1.977 | 0.11 | 19 |
| 18 | [PtIICl(4-Me-10-tBuBPI) (120) | 2.055 | 1.966 | 0.12 | 81 |
Fig. 8 shows a good correlation between tetrahedricity factor (Φ) and endocyclic amine–palladium distances. The increasing central Nind–Pd distance is in agreement with the degree of distortion that is nearly negligible in 107 and the highest in 103. The unique structure of 115 and the N,N,S coordination of 114 likely explain their deviation from the well-observed trend.
The small structural differences of platinum complexes resulted in similar Φ values with 5% deviation (Fig. 8 inset). Distorting factors have a minimal influence on the structures so the trend can be considered zero. This indicates that neither anions nor ligands–coligands can influence the electronic structure of Pt2+.
Fig. 9 shows how tetrahedricity factor (Φ) has been calculated in case of palladium complexes from the opposite planes of a molecule. The method was described on Scheme 3 however the diversity of Pd complexes allows an expressive representation. The angle between planes 1–2 (ϕ1) and planes 3–4 (ϕ2) is small as expected for square planar complexes and increases as distortion grows towards the tetrahedral form. The largest angle (ϕ0) is close to 90° for both SP-4 and T-4 in an ideal case.
3.4. Complexes of 1B metals
From the group of 1B elements only copper containing molecules have been synthesized with BIIs (Table 8). Octahedral, trigonal-bipyramidal, furthermore the transition between tetrahedral and square-planar geometries appear in various structures. Similarly to manganese a large number of enzymes contain copper as co-factor. Model complexes of SOD, quercetinase or catechol oxidase are known to have considerable mimicking activities.| Ligand | Complex | NPy–Cua (Å) | Nind–Cub (Å) | τ (Φ) | Ref. |
|---|---|---|---|---|---|
| a The average pyridyl N–Cu distance.b The isoindoline N(H)–Cu distance. | |||||
| 7 | [CuII(BPIH)(NCCH3)(OClO3)] (121) | 2.020 | 1.911 | 0.21 | 46 |
| 7 | [CuII(4-MeBPI)(OAc)] (122) | 2.019 | 1.884 | — | 88 |
| 7 | [CuII(ind)(pga)(H2O)] (123) | 2.027 | 1.902 | 0.21 | 47 |
| 7 | [CuII(ind)(mco)] (124) | 2.041 | 1.905 | 0.38 | 89 |
| 7 | [CuII(NBAII)(OAc)] (125) | 2.003 | 1.895 | — | 90 |
| 8 | [Cu2II(4′Me-ind)2(MeOH)(μ-OH)]ClO4 MeOH (126) | 2.017 | 1.920, 1.906 | 0.36 | 91 |
| 8 | [CuII(4′Me-ind)(H2O)2]ClO4 (127) | 1.979 | 1.904 | 0.66 | 92 |
| 9 | [CuII(bimind)(pga)] 2 DMF (128) | 1.947 | 1.934 | — | 47 |
| 9 | [CuII(bimind)(ba)] DMF (129) | 1.950 | 1.931 | — | 47 |
| 9 | [CuII(bimind)OAc] (130) | 1.967 | 1.949 | — | 66 |
| 10 | [CuII(BTI)(OAc)] (131) | 1.968 | 1.960 | — | 88 |
| 10 | [CuII(BTI)2] (132) | 2.181 | 2.001, 1.983 | — | 46 |
| 11 | [CuII(3′Me-ind)2] (133) | 2.265 | 1.952, 1.948 | — | 46 |
| 13 | [CuII(Mebimind)(Cl)]·0.5CH2Cl2 (134) | 1.956 | 1.945 | 0.44 | 46 |
| 15 | [CuII(benzBTI)(Cl)] C7H8 (135) | 1.987 | 1.928 | 0.48 | 46 |
| 31 | [CuII(tetraphenyl-pinBPI)(OAc)] (136) | 2.094 | 1.883 | — | 13 |
| 32 | [CuII(Me-pentBPI)(OAc)] (137) | 2.038 | 1.912, 1.905 | — | 93 |
| 33 | [CuII(Bn-pentBPI)(OAc)] (138) | 2.050 | 1.898 | — | 93 |
| 32 | [CuII(OMe-pentBPI)(Cl)] (139) | 2.029 | 1.913 | 0.36 | 93 |
| 33 | [CuII(Bn-pentBPIH)(CF3SO3)2] (140) | 1.941 | 1.902 | 0.52 | 93 |
| 33 | [CuII(Bn-pentBPI)(CF3SO3)] H2O (141) | 1.932 | 1.887 | 0.58 | 93 |
Cu(3′MePyOIND)2 (142) (Fig. 10) belongs to the unsyBII family with a tetrahedral arrangement of ligands around the copper center.23 The complex was found to exhibit catecholase activity by catalyzing the conversion of 3,5-di-tert-butylcatechol to 3,5-di-tert-butylbenzoquinone. Same activity and similar catalytic strength was found for 126 however their coordination sphere is different. Certainly, an incomplete third arm on BII gives the advantage of tunable (hydrophilicity, hydrophobicity, chirality, etc.) 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes while enough space remains for substrate accessibility. Other unsymmetric bidentate isoindoline compounds have been reported to possess antiproliferative activity (143, 144).10
1 complexes while enough space remains for substrate accessibility. Other unsymmetric bidentate isoindoline compounds have been reported to possess antiproliferative activity (143, 144).10
 | ||
| Fig. 10 UnsyBII ligands and a complex with copper.10,23 | ||
Stability and redox inactivity have been proved of OC-6 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes (132, 133 and derivatives with ligands: 7, 8, 9, 13, 15). Their 1
1 complexes (132, 133 and derivatives with ligands: 7, 8, 9, 13, 15). Their 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 counterparts (121, 134, 135 and derivatives with the same ligands) effectively participated in superoxide scavenging reactions mimicking SOD enzymes.46
1 counterparts (121, 134, 135 and derivatives with the same ligands) effectively participated in superoxide scavenging reactions mimicking SOD enzymes.46
3.5. Complexes of 2B metals
Metals of group 2B or group 12 are considered highly toxic except zinc that is contrarily among the most important metals in public health. Enzymes with zinc active center are widespread in living organisms therefore deficiency may lead to growth retardation while excess of it unsettles the sensitive biogenic metal balance.The stable d10 electron configuration of Zn2+ has a strong impact on complex formation with BIIs. Conventional methods like using triethylamine base in order to deprotonate the ligand are usually insufficient.69,92,94 The two structures of 147 and 148 (Table 9) have been obtained with tetra-n-butylammonium hydroxide, although some parts in 148 may have remained nondeprotonated.94 151 and 152 have been synthesized in situ from the ligand precursor using the metal salt as catalyst.
| Ligand | Complex | NPy–Zna (Å) | Nind–Znb (Å) | τ | Ref. |
|---|---|---|---|---|---|
| a The average pyridyl N–Zn distance.b The isoindoline N(H)–Zn distance. | |||||
| 7 | [ZnII(ind)2] (145) | — | — | — | 21 |
| 7 | [ZnII(NBAII)(OAc)] (146) | — | — | — | 90 |
| 8 | [ZnII3(4′Me-ind)4] (ClO4)2·5H2O (147) | 2.020 | 1.930 | — | 92 |
| 8 | [ZnII(4′Me-ind)2] (148) | 2.243 | 2.053, 2038 | — | 94 |
| 9 | [ZnII(bimind)OAc DMF] (149) | 2.083 | 2.064 | — | 66 |
| 10 | [ZnII(BTI)(OMe)3] (150) | 2.094 | 2.056 | — | 59 |
| 20 | [ZnII(phbox)2] (151) | 2.168 | 2.121 | — | 95 |
| 34 | [ZnII(iPrbox)2] (152) | 2.170 | 2.174 | — | 95 |
The lack of complete deprotonation occurred in 155 (Table 10). The flexibility of the isoindoline system allows the shuttling of the iminium proton between imine N atoms. This intramolecular proton transfer was followed by 1H NMR spectroscopy.94,97 The three Cd structures (153–155) with their preparative circumstances perfectly represent the coordinative capacities of monoanionic aroBIIs.
The last paper to be overviewed is an individual work on a mercury compound by Wicholas' group. The coordination pattern of Hg resembles 153 that is not surprising because similar reaction conditions have been applied. The outer sphere solvent and the inner sphere anion coligand are the dissimilarities [Hg3II(4′Me-ind)4] (NO3)2 4 MeOH.96
As a closure of this section regularities in complex stability have been drafted with complex series of the same ligands. The stability of isoindoline complexes is rarely determined by the K constant. Fortunately, a stability order was found by Irving and Williams for high-spin complexes of divalent metal ions in the middle of the previous century.98 The combination of knowledge in Jahn–Teller effect, crystal field stabilization energy and ionic radius is the key to explain the series. Since it stands for a wide variety of ligands, it was found to be suitable for ligands 7 and 10 as well (Fig. 11). Bond distances of isoindoline Nind–M2+ and NPy–M2+ show the same pattern with the Irving-Williams series.
3.6. Complexes of aminophthalazines (PAP)
Metal chelating capacity and the simplicity of synthesis makes PAPs excellent “isoindoline-based” ligands. The most eye-catching difference from isoindolines is the vicinal N atoms that determine the structural arrangement of metals creating a perfect environment for bi-, or trinuclear complexes. The bidentate coordination and the proximity of metal ions forms oxo-, halogenato or even azido-bridges in several occasions. Thompson et al. presented the first synthesis of PAP14 in 1969 and focused on nickel, copper and cobalt complexes later on in his work (Table 11).102–105 The only manganese complex has been characterized by spectroscopic methods and elemental analysis suggesting the [MnII2(6′Me2PAP)2Cl2] formula.99| Ligand | Complex | M–M (Å) | Nind–M (Å) | Ref. |
|---|---|---|---|---|
| 7 | [CuII2(PAP)Cl3(OH)]·1.5H2O (156) | — | 2.000–2.038 | 14 and 100 |
| 7 | [FeII2(μ-OMe)2(PAP)Cl4]·2MeOH (157) | 3.021 | 2.204 | 101 |
| 7 | [CoII2(PAP)3(OH)2]Br4·9H2O (158) | 2.795 | 1.880 | 102 |
| 7 | [NiII2(PAP)3(H2O)2]Br4·6H2O (159) | 3.390 | 2.075, 2.084 | 102 |
| 35 | [CuII2(PAP-4,6Me)4Cl4] (160) | 3.251 | 2.022 | 103 |
| 7 | [CuII4(PAP)2(μ2-1,1-N3)2(μ2-1,3-N3)2(μ2-CH3OH)2(N3)4] (161) | 3.207 | 2.021 | 104 |
| 11 | [CuII4(PAP-3Me)2(μ2-1,1-N3)2(μ2-1,3-N3)2(H2O)2(NO3)2] (162) | 3.163 | 2.009, 1.970 | 104 |
| 7 | [NiII(PAPH)2(H2O)2](BF4)4 (163) | — | 2.064 | 105 |
| 7 | [NiII2(PAP)3(H2O)2](NO3)4·5H2O (164) | 3.126 | 2.070, 2.059 | 105 |
| 7 | [CoIII2(PAP)3(OH)2](NO3)4·xH2O (165) | 2.777 | 1.89, 1.91 | 105 |
| 7 | [NiII3(PAP)3(OH)2](BF4)3F·xH2O (166) | 2.997–3.026 | 2.048–2.057 | 105 |
| 13 | [FeIII3(MebimPAP)3O]+ (167) | 3.305–3.33 | 2.047–2.066 | 106 |
| 14 | [FeIII2(μ-OMe)2(5MeBTI-PAPH2)Cl4] (168) | 3.012 | 2.249 | 68 |
4. Applications, aims of development
In approximately 50–60% of the scientific papers in this subject new molecules are presented only with the purpose of structural characterization. Fundamentally, one has to know about the N,N,N donor title ligands that they are promising carriers of all transition metals leaving their redox properties almost intact. Their tunability is not only a basic requirement but based on the number of publications and the wide variety of new applications found it is reality as well. An aging review on pincer-type complexes107 pointed the focus on light-harvesting, sensoring and biological approaches in its closing remarks. These remarks are still valid. The increasing need for energy, the development of modern devices and the improvement of human healthcare still fully cover those suggestions in our modern life.With some perspicuous intention the current major research directions will be discussed in two broader categories such as biomimetics and chemical catalysis.
4.1. Biomimetic studies
Oxidoreductases is a fairly populous subgroup of enzymes that can perform electron or hydrogen atom transfer reactions between the interacting molecules. Some of them with metal content (metalloenzymes) are phenoxazinone synthase (EC 1.10.3.4), catalase (EC 1.11.1.6), catechol oxidase (EC 1.10.3.1), catechol dioxygenase (EC 1.13.11.1-3), methane monooxygenase (EC 1.14.13.25) and superoxide dismutases (EC 1.15.1.1). These representatives are the subjects of continuous research on enzyme mimics related to isoindoline-based bioactive complexes.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε = 4.20). The general goal of enzyme mimics is not the development towards perfect therapeutics but to have better insight into the actual mechanism. Although, copper is the active metal in PHS, models have been created with cobalt,112 iron113 and even manganese114 complexes. The corresponding isoindoline-based compounds have been collected in Table 12. Their catalytic efficacy (kcat/KM) complies with the other models found in the literature of this topic, furthermore straight correlation was observed between oxidation potential of the iron center in the precursor complex and catalytic efficacy.
ε = 4.20). The general goal of enzyme mimics is not the development towards perfect therapeutics but to have better insight into the actual mechanism. Although, copper is the active metal in PHS, models have been created with cobalt,112 iron113 and even manganese114 complexes. The corresponding isoindoline-based compounds have been collected in Table 12. Their catalytic efficacy (kcat/KM) complies with the other models found in the literature of this topic, furthermore straight correlation was observed between oxidation potential of the iron center in the precursor complex and catalytic efficacy.
Two possible mechanisms have been proposed as overall conclusion of PHS mimics with isoindoline complexes. One of them is a metal-based oxidation shown as route 1 in Scheme 4. The rate determining step is the ternary complex formation of metal, ligand and dioxygen that is facilitated by the dioxygen–metal interaction. In the second route a radical formation from H2O2 is assumed initiated by the complexes but substrate transformation happens via standard radical pathway. The first available model experiments were carried out by Prati et al.109 and Barry et al.110 around the 1990's. The recent knowledge is still based on the original 3 × 2e− oxidation process however, it has been extended to other metals and with some radical involvement.
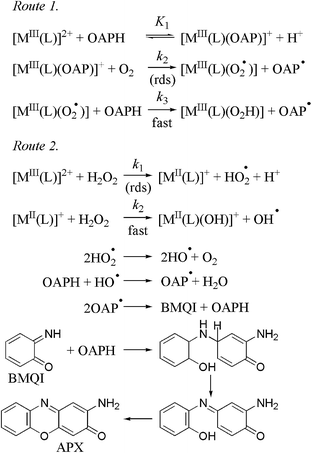 | ||
| Scheme 4 The proposed mechanism of phenoxazinone synthase model reactions.67 | ||
Recently, as a major improvement an expressive correlation was discovered (Fig. 12) how fine-tuning of the precursor complex could increase reaction rates during AXP synthesis. Modifications on BII strongly influence the oxidation potential of iron complexes that has a straight effect on the reaction rates.
 | ||
| Fig. 12 Isoindoline dependent oxidation potentials in iron complexes vs. reaction rates in phenoxazinone synthase mimics.67 | ||
According to kinetic data a ternary complex formation is plausible among superoxoiron(III), the corresponding ligand and dioxygen (route 1, step 2 in Scheme 4) in the rate determining step.67
The catalase activity of manganese complexes has been determined by gas-volumetric method measuring dioxygen evolution from H2O2 in specific time intervals. Results are shown in Table 13 compared to the original enzyme efficiency from various sources. The kcat/KM values of models are significantly lower than catalase, but they are in the same range with other model compounds.117 The most active compound up to this date is a Mn4+ complex with a SALPN type ligand that is still far behind the native enzyme.118 The hypothetical cycle of mechanism is explained by Mn3+–Mn4+ (mononuclear) or Mn2+/Mn2+–Mn3+/Mn3+ (dinuclear) transitions.118,119
Considering future directions of developments some useful observations have been made how coligands and other structural changes are able to influence activity. Inactive 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 or slightly active 1
1 or slightly active 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (metal–ligand ratio) manganese complexes could be enhanced by active molecules such as TEMPO (2,2,6,6-tetramethyl-piperidin-1-yl-oxyl)120 or different N-donor heterocycles (pyridine, imidazole)17 (L). Electron-donating moieties on both coligands (changing imidazole to 1-methylimidazole)17 and R-groups of 3 (more effective 9 than 7)50 have increasing effect on activity. On the basis of reaction kinetic data a mechanism has been proposed where Mn(II) is transformed into a Mn(IV)
2 (metal–ligand ratio) manganese complexes could be enhanced by active molecules such as TEMPO (2,2,6,6-tetramethyl-piperidin-1-yl-oxyl)120 or different N-donor heterocycles (pyridine, imidazole)17 (L). Electron-donating moieties on both coligands (changing imidazole to 1-methylimidazole)17 and R-groups of 3 (more effective 9 than 7)50 have increasing effect on activity. On the basis of reaction kinetic data a mechanism has been proposed where Mn(II) is transformed into a Mn(IV)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O species that is reduced by H2O2 to its initial form at the end of the catalytic cycle (Scheme 6).17
O species that is reduced by H2O2 to its initial form at the end of the catalytic cycle (Scheme 6).17
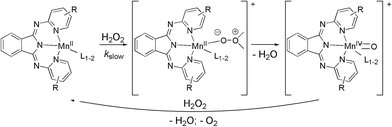 | ||
| Scheme 6 The proposed mechanism of catalase model reactions.17 | ||
McCord–Fridovich assay125 is a well-known spectrophotometric protocol that is used to determine superoxide dismutase activity. All complexes presented in Table 14 have been tested in a reaction of nitroblue tetrazolium (NBT) or cytochrome c reduction. The superoxide radical anion (O2˙−) was generated in situ by the xanthine/xanthine oxidase reaction, and its decomposition was monitored spectrophotometrically by following the formation of diformazan from NBT at 560 nm, or alternatively, by monitoring the reduction of cytochrome c at 550 nm. The products of the disproportionation are dioxygen and hydrogen peroxide.
| Ligand | Complex | Epca (Eox/red) | IC50 (10−6 M) | kMcF (kcat) (10−6 M−1 s−1) | Ref. |
|---|---|---|---|---|---|
| a Redox potential values are expressed in Volt. | |||||
| — | CuZnSOD | — | — | 2000 | 126 |
| 9 | [CuII(bimindH)(NCCH3)(OClO3)]ClO4 (171) | −0.835 | 6.62 | 1.98 | 46 |
| 9 | [CuII(bimindH)Cl2] (172) | −0.835 | 10.99 | 1.19 | 46 |
| 13 | [CuII(Mebimind)Cl]·0.5CH2Cl2 (134) | −0.860 | 27.37 | 0.48 | 46 |
| 10 | [CuII(BTIH)(NCCH3)(OClO3)]ClO4 (173) | −0.826 | 4.69 | 2.80 | 46 |
| 10 | [CuII(BTI)Cl] (174) | −0.826 | 7.19 | 1.83 | 46 |
| 7 | [CuII(BPIH)(NCCH3)(OClO3)]ClO4 (121) | −0.724 | 0.95 | 13.82 | 46 |
| 7 | [CuII(indH)Cl2] (175) | −0.724 | 2.06 | 6.37 | 46 |
| 15 | [CuII(benzBTI)Cl] (135) | −0.650 | 1.21 | 10.85 | 46 |
| 10 | [FeII(BTI)2] (176) | (0.555) | — | (5.00) | 22 |
| 10 | [FeII–III(BTI)2] MeCN (55) | — | — | (5.28) | 22 |
| 7 | [FeII(ind)2] (47) | (0.319) | — | (3.83) | 22 |
| 8 | [FeII(4′Me-ind)2] (177) | (0.265) | — | (3.62) | 22 |
| 9 | [FeII(bimind)2] (52) | (0.205) | — | (3.06) | 22 |
| 13 | [FeII(Mebimind)2] (178) | (0.123) | — | (2.50) | 22 |
| 9 | [MnII(bimind)2] (179) | (−0.021) | 3.10 | (3.28) | 15 |
| 13 | [MnII(Mebimind)2] (180) | (0.005) | 6.36 | (1.60) | 15 |
| 10 | [MnII(BTI)2] (45) | (0.082) | 12.10 | (0.83) | 15 |
| 7 | [MnII(ind)2] (181) | (0.015) | 8.74 | (1.26) | 15 |
| 11 | [MnII(3′Me-ind)2] (182) | (−0.098) | 11.10 | (0.99) | 15 |
| 8 | [MnII(4′Me-ind)2] (183) | (−0.082) | 9.44 | (1.08) | 15 |
| 7 | [CoII(ind)2] (184) | −0.483 | 4.29 | 2.35 | 53 |
| 11 | [CoII(3′Me-ind)2] (185) | −0.512 | 4.82 | 2.10 | 53 |
| 8 | [CoII(4′Me-ind)2] (186) | −0.583 | 15.90 | 0.64 | 53 |
SOD models are categorized by inhibition constant IC50 values (nM to μM range, the concentration where inhibition of NBT or cyt c reduction is 50%). Unlike with other enzyme models the primary aim here is the invention of an effective SOD substitute. Kinetic measurements focus on the achievement of lower IC50 or higher kMcF instead of the understanding of the mechanism. Rather new findings with cobalamins may point to the right direction namely their efficacy approach SOD.127
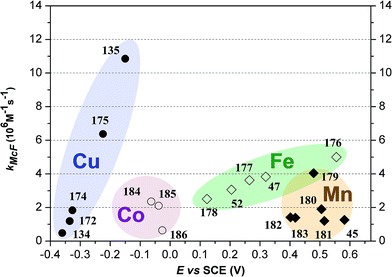
Overviewing the results of SOD mimicking activity it is clear that the transition metal complexes of BIIs are relevant artificial models of SOD function. Redox potentials, coordination geometry and ligands have the key roles in the process. Octahedral complexes with irreversible redox behavior have a little contribution to superoxide destruction.46,53 Scenic correlation was found between reduction peak potential (Epc) and inhibition constant at copper complexes.46 Iron activities correlate better with redox potentials (Eox/red)22 and manganese complexes are influenced by counter-anions and non-coordinating ligand parts.15 Certainly, the redox properties of metals show the major influence that was also proved by comparing superoxide scavenging reaction of Mn, Fe, Co and Ni complexes of the identical ligand (7). The highest activity of Fe was observed at that time (Fig. 13).53 Although, the series is not complete (missing data for homoleptic complex of Cu) it is clear that iron and copper have promising values at least among the SOD models (see also Fig. 14).
 | ||
Fig. 13 Rate constants for the superoxide scavenging reaction of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio (metal–ligand) complexes of 7.53 2 ratio (metal–ligand) complexes of 7.53 | ||
Fig. 14 shows the kMcF rate constants plotted against the E°′1/2 for all complexes that have been investigated as SOD models. A clear trend is observable in case of Cu and Fe while Mn and Co act differently. Redox potential values can be set where SOD activity is optimal. These are around the CuZnSOD redox couple (∼0.06 V vs. Ag/AgCl in 1 M KCl at pH 7.5 in HEPES buffer)128 and above the O2/O2˙− potential (−0.40 V vs. SCE – saturated calomel electrode).129–131
The N-donor coordination sphere built by BIIs is an accurate model environment of CuZnSOD's copper, where three histidine ligands surround that part of the active center. As it was mentioned earlier cobalamins127 can approach SOD activity implying to further ligand effects that have to be taken into consideration for future modelling studies.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
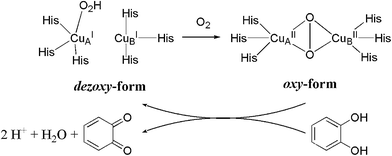
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) η2 form. A cyclic pathway between dezoxy (inactive) and oxy (active) form of the enzyme is the driving force of catechol oxidation as it is depicted in Scheme 7.
η2 form. A cyclic pathway between dezoxy (inactive) and oxy (active) form of the enzyme is the driving force of catechol oxidation as it is depicted in Scheme 7.
Model experiments are usually carried out with 3,5-di-tert-butylcatechol to avoid dimerization. Products are 3,5-di-tert-butyl-1,2-benzoquinone (3,5-DTBQ) and H2O2. As it is expected from model systems the activities are several magnitudes lower compared to the enzyme. The slow transformation is fortunate for reaction kinetic observations. The kcat/KM and TON values of Table 15 give the general information on substrate conversion during time unit. Turnover rate differences show that binuclear PAP complex have better mimicking properties at the same time mononuclear BIIs are more suitable for the investigations of influential factors. The only binuclear copper complex (126) is the most accurate catecholase structural model. The Cu–Cu distance is 3.274 Å that makes catechol coordination possible.
| Ligand | Complex | TON (h−1) | kcat/KM (M−1 s−1) | Ref. |
|---|---|---|---|---|
| — | Catechol oxidase from I. batatas | — | 8250 | 133 |
| 12 | [Mn2(6′Me2PAP)2Cl4] (187) | 167.9 | 7.25 | 99 |
| 12 | [Mn(6′Me2indH)(H2O)2(CH3CN)](ClO4)2 (188) | 48.8 | 1.07 | 16 |
| 7NMe | [CuI(Me2ind)I2] (189) | 65.0 | 67.40 | 33 |
| 8 | [Cu2II(4′Me-ind)2(MeOH)(μ-OH)]ClO4 MeOH (126) | — | 0.749 | 91 |
| 11 | [Cu(3′MePyOIND)2] (142) | 21.5 | 23.37 | 23 |
According to the enzymatic met (native) and oxy form the ideal distance has to be between 2.9 Å and 3.8 Å. The enzyme like behavior of models is attributed to the binuclear cores (with the appropriate distances) and to the presence of enzyme-like N-donor environment (Fig. 15).
 | ||
| Fig. 15 Catechol oxidase active site (left), the coordination environment of [Cu2II(4′Me-ind)2(MeOH)(μ-OH)]ClO4 MeOH (126) (middle)91 and the proposed structure of [Mn2(6′Me2PAP)2Cl4] (187) (right).99 | ||
Observations from the kinetic experiments imply to a biocatalytic circle where a ternary complex of dioxygen, substrate and catalyst is involved, furthermore bases and electron donating potential of the substrate have an effect on the mechanism. Basicity is considerable from both catalyst and external side as the prior deprotonation of the catechol is required for the activation of the “dezoxy form” of the catalyst.16,23
 | ||
| Scheme 8 The proposed mechanism of catechol 1,2-dioxygenase model reactions (top) and the effect of fine-tuning on the k reaction rate (bottom).65 | ||
The reaction mechanism of catechol dioxygenase mimics hypothesize certain active iron–oxygen species that can materialize primarily from iron(III). In the most recent works of our group we had special attention on stable 1,2-peroxodiiron(III) intermediates.34,68 These species have been identified in several industrially promising enzymes (e.g. soluble methane monooxygenase, ribonucleotide reductase or desaturases) by Raman and EXAFS spectroscopy,134,135 furthermore by characteristic UV-Vis vibrations at 600–750 nm closer to the near-infrared region. Oxidation reactions of thioanisoles and benzyl alcohols have been investigated in the presence of H2O2 using mild reaction conditions aiming for selectively oxidized products. The work presented in these papers contains proofs of a metal-based oxidant with the clear exclusion of hydroxyl radicals. Reactions with para-substituted reagents showed changes in oxidation rate as a function of the nature of the para substituent. The negative Hammett ρ values were indicative of an electrophilic oxidant, furthermore KIE (kinetic isotope effect) supported the theory about the lack of radical involvement. Results are a part of a continuous research on the role of metal-based oxidants in non-heme diiron enzymes.
4.2. Catalysis; chemical, quantum chemical and biological applications
AroBII ligands possess undoubtedly perfect properties for complex formation. Structural properties, coordination modes and unexpected molecular configurations are presented by the majority of scientific papers. Better understanding in basic features leads to new ideas for applications that show a growing tendency year by year.Palladacycles are known with unsyBIIs as well.136,137 Caryl–H bond activation was observed in this case.
Catalytic hydrogenation with palladium has been carried out with more efficacy on aliphatic olefins.18 It is notable that the catalyst could be reused in several cycles.
Multiple papers from L. H. Gade's group describe C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond activation. The peroxylation of cyclohexene to tert-butylperoxy-3-cyclohexene has been tested with copper, cobalt and iron complexes.61,88 Selectivity towards the desired product was reported to be outstanding. Using highly reactive oxaziridine type oxidizing agents even epoxidation products have been detected from a great number of olefins by iridium-aroBIIs (Scheme 9).78
C bond activation. The peroxylation of cyclohexene to tert-butylperoxy-3-cyclohexene has been tested with copper, cobalt and iron complexes.61,88 Selectivity towards the desired product was reported to be outstanding. Using highly reactive oxaziridine type oxidizing agents even epoxidation products have been detected from a great number of olefins by iridium-aroBIIs (Scheme 9).78
Cobalt(III)–alkylperoxy complexes can be activated by high energy light irradiation. Oxidation products of alkanes or alkenes are alkylperoxides or ketones/alcohols depending on the presence of inert atmosphere.138
Quantum yields dramatically change when luminescence of complexes is investigated. It dropped below 5% when platinum or rare earth metals have been measured.19,32,46
Specific optical properties have a wide range of applications in modern electronic devices. Crystallographic direction dependent refractive index (birefringence) has been determined for a few aroBII ligands. These values describe the 3D structure of a crystalline material giving information on anisotropic polarizability or supramolecular orientation, etc.139
UnsyBIIs have already been discussed (see above) in their active palladacycle form and as bidentate N-donor ligands. Recently, antiproliferative activity has been tested too on five human tumor cell lines. The results serve as guideline for future development of bioactive isoindoline derivatives.10
5. Perspectives
The approximately six decades long history of bis-iminoisoindoline derivatives and complexes led to numerous discoveries in their chemistry and biochemistry. Now, with precise insight into all structural features and with all the improved techniques in synthesis there is a great opportunity to further exploit their capabilities. There is just and endless possibility to attach various sidearms on DII cores that control enantioselective, anionic and coordinative behavior. Fine-tuning of these ligands is simply prominent. Photophysical properties both by crystallographic means and ligand based should be taken into consideration for research directions. In catalysis, the pincer-type coordination defines two major trends. 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes are successfully applied on several fields due to the accessibility of the metal center while octahedral 2
1 complexes are successfully applied on several fields due to the accessibility of the metal center while octahedral 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes are limited to specific interactions. The greatly promising achievements in enzyme mimics have significance in modern drug development.
1 complexes are limited to specific interactions. The greatly promising achievements in enzyme mimics have significance in modern drug development.
Acknowledgements
Financial support of the Hungarian National Research Fund (OTKA K108489), TÁMOP-4.2.2.A-11/1/KONV-2012-0071, TÁMOP-4.2.2/B-10/1-2010-0025, and COST CM1003 are gratefully acknowledged.Notes and references
- J. A. Elvidge and R. P. Linstead, J. Chem. Soc., 1953, 5000 Search PubMed
.
- P. F. Clark, J. A. Elvidge and R. P. Linstead, J. Chem. Soc., 1953, 3593 RSC
.
- W. O. Siegl, Inorg. Chim. Acta, 1977, 25, L65 CrossRef CAS
.
- R. R. Gagné, R. S. Gall, G. S. Lisensky, R. E. Marsh and L. M. Speltz, Inorg. Chem., 1979, 18, 771 CrossRef
.
- R. R. Gagné, W. A. Marritt, D. N. Marks and W. O. Siegl, Inorg. Chem., 1981, 20, 3260 CrossRef
.
- A. W. Addison, P. J. Burke and K. Henrick, Inorg. Chem., 1982, 21, 60 CrossRef CAS
.
- W. O. Siegl, J. Org. Chem., 1977, 42, 1872 CrossRef CAS
.
- G. de la Torre, P. Vázquez, F. Agulló-López and T. Torres, Chem. Rev., 2004, 104, 3723 CrossRef CAS PubMed
.
- H. Alivan and J. E. Lier, Porphyrins and Phthalocyanines as Photosensitizers and Radiosensitizers in Handbook of Porphyrin Science, ed. K. M. Kadish, K. M. Smith and R. Guilard, World Scientific Publishing, Singapore, 2010, vol. 4, pp. 1–99 Search PubMed
.
- I. Sović, V. Stilinović, B. Kaitner, S. Kraljević-Pavelić, M. Bujak, K. Čuljak, P. Novak and G. Karminski-Zamola, J. Mol. Struct., 2011, 1006, 259 CrossRef PubMed
.
- G. Cignarella and P. Sanna, J. Med. Chem., 1981, 24, 1003 CrossRef CAS
.
- S. Klutchko, C. J. Blankley, R. W. Fleming, J. M. Hinkley, A. E. Werner, I. Nordin, A. Holmes, M. L. Hoefle, D. M. Cohen, A. D. Essenburg and H. R. Kaplan, J. Med. Chem., 1986, 29, 1953 CrossRef CAS
.
- B. K. Langlotz, H. Wadepohl and L. H. Gade, Angew. Chem., Int. Ed., 2008, 47, 4670 CrossRef CAS PubMed
.
- L. K. Thompson, V. T. Chacko, J. A. Elvidge, A. B. P. Lever and R. V. Parish, Can. J. Chem., 1969, 47, 4141 CrossRef CAS
.
- J. S. Pap, B. Kripli, T. Váradi, M. Giorgi, J. Kaizer and G. Speier, J. Inorg. Biochem., 2011, 105, 911 CrossRef CAS PubMed
.
- J. Kaizer, G. Barath, R. Csonka, G. Speier, L. Korecz, A. Rockenbauer and L. Párkányi, J. Inorg. Biochem., 2008, 102, 773 CrossRef CAS PubMed
.
- J. Kaizer, T. Csay, P. Kövári, G. Speier and L. Párkányi, J. Mol. Catal. A: Chem., 2008, 280, 203 CrossRef CAS PubMed
.
- B. Siggelkow, M. B. Meder, C. H. Galka and L. H. Gade, Eur. J. Inorg. Chem., 2004, 3424 CrossRef CAS
.
- H.-M. Wen, Y.-H. Wu, Y. Fan, L.-Y. Zhang, C.-N. Chen and Z. -N. Chen, Inorg. Chem., 2010, 49, 2210 CrossRef CAS PubMed
.
- I.-S. Tamgho, J. T. Engle and C. J. Ziegler, Tetrahedron Lett., 2013, 54, 6114 CrossRef CAS PubMed
.
- P. J. Domaille, R. L. Harlow, S. D. Ittel and W. G. Peet, Inorg. Chem., 1983, 22, 3944 CrossRef CAS
.
- B. Kripli, G. Baráth, É. Balogh-Hergovich, M. Giorgi, A. J. Simaan, L. Párkányi, J. S. Pap, J. Kaizer and G. Speier, Inorg. Chem.
Commun., 2011, 14, 205 CrossRef CAS PubMed
.
- J. Kaizer, T. Csay, G. Speier and M. Giorgi, J. Mol. Catal. A: Chem., 2010, 329, 71 CrossRef CAS PubMed
.
- C. G. Claessens, U. Hahn and T. Torres, Chem. Rec., 2008, 8, 75 CrossRef CAS PubMed
.
- A. B. Sorokin, Chem. Rev., 2013, 113, 8152 CrossRef CAS PubMed
.
- P. F. Clark, J. A. Elvidge and J. H. Golden, J. Chem. Soc., 1956, 4135 RSC
.
- H. A. Luts, J. Pharm. Sci., 1969, 58, 496 CrossRef CAS
.
- R. Sato, T. Senzaki, Y. Shikazaki, T. Goto and M. Saito, Chem. Lett., 1984, 1423 CrossRef CAS
.
- R. Sato, M. Nakayama, Y. Yuzawa, T. Goto and M. Saito, Chem. Lett., 1985, 1887 CrossRef CAS
.
- V. V. Negrebetskii, O. V. Balitskaya and M. Y. Kornilov, Russ. J. Gen. Chem., 1983, 53, 2320 Search PubMed
.
- M. N. Bochkarev, T. V. Balashova, A. A. Maleev, I. I. Pestova, G. K. Fukin, E. V. Baranov and Y. A. Kurskii, Russ. Chem. Bull. Int. Ed., 2008, 57, 2162 CrossRef CAS PubMed
.
- A. A. Maleev, T. V. Balashova, G. K. Fukin, M. A. Katkova, M. A. Lopatin and M. N. Bochkarev, Polyhedron, 2010, 29, 10 CrossRef CAS PubMed
.
- Á. Kupán, J. Kaizer, G. Speier, M. Giorgi, M. Réglier and F. Pollreisz, J. Inorg. Biochem., 2009, 103, 389 CrossRef PubMed
.
- J. S. Pap, M. A. Cranswick, É. Balogh-Hergovich, G. Baráth, M. Giorgi, G. T. Rohde, J. Kaizer, G. Speier and L. Que Jr, Eur. J. Inorg. Chem., 2013, 3858 CrossRef CAS PubMed
.
- N. C. Fletcher, F. R. Keene, M. Ziegler, H. Stoeckli-Evans, H. Viebrock and A. von Zelewsky, Helv. Chim. Acta, 1996, 79, 1192 CrossRef CAS
.
- Q.-H. Dang, H. Wadepohl and L. H. Gade, Chem.–Eur. J., 2011, 17, 14922 CrossRef PubMed
.
- D. C. Sauer, H. Wadepohl and L. H. Gade, Inorg. Chem., 2012, 51, 12948 CrossRef CAS PubMed
.
- M. Bröring and C. Kleeberg, Inorg. Chim. Acta, 2009, 362, 1065 CrossRef PubMed
.
- B. L. Dietrich, J. Egbert, A. M. Morris and M. Wicholas, Inorg. Chem., 2005, 44, 6476 CrossRef CAS PubMed
.
- M. Bröring and C. Kleeberg, Dalton Trans., 2007, 1101 RSC
.
- M. Bröring, C. Kleeberg and E. C. Tejero, Eur. J. Inorg. Chem., 2007, 3208 CrossRef
.
- M. Bröring, C. Kleeberg and S. Köhler, Inorg. Chem., 2008, 47, 6404 CrossRef PubMed
.
- C. Kleeberg and M. Bröring, Polyhedron, 2010, 29, 507 CrossRef CAS PubMed
.
- A. W. Addison and T. N. Rao, J. Chem. Soc., Dalton Trans., 1984, 1349 RSC
.
- M.-A. Munoz-Hernandez, T. S. Keizer, P. Wei, S. Parkin and D. A. Atwood, Inorg. Chem., 2001, 40, 6782 CrossRef CAS PubMed
.
- J. S. Pap, B. Kripli, V. Bányai, M. Giorgi, L. Korecz, T. Gajda, D. Árus, J. Kaizer and G. Speier, Inorg. Chim. Acta, 2011, 376, 158 CrossRef CAS PubMed
.
- J. S. Pap, V. Bányai, D. S. Szilvási, J. Kaizer, G. Speier and M. Giorgi, Inorg. Chem. Commun., 2011, 14, 1767 CrossRef CAS PubMed
.
- É. Balogh-Hergovich, G. Speier, M. Réglier, M. Giorgi, E. Kuzmann and A. Vértes, Inorg. Chem. Commun., 2005, 8, 457 CrossRef PubMed
.
- J. Kaizer, G. Baráth, G. Speier, M. Réglier and M. Giorgi, Inorg. Chem. Commun., 2007, 10, 292 CrossRef CAS PubMed
.
- J. Kaizer, B. Kripli, G. Speier and L. Párkányi, Polyhedron, 2009, 28, 933 CrossRef CAS PubMed
.
- J. S. Pap, M. Giorgi, J. Kaizer and G. Speier, Inorg. Chem. Commun., 2013, 27, 152 CrossRef CAS PubMed
.
- A. Yashima, T. Matsuda, T. Sato, M. Takahashi and K. Sakai, U.S. Pat. 4 568 769 to Sumitomo, 1986
.
- J. S. Pap, B. Kripli, M. Giorgi, J. Kaizer and G. Speier, Transition Met. Chem., 2011, 36, 481 CrossRef CAS
.
- K. Hanson, N. Patel, M. T. Whited, P. I. Djurovich and M. E. Thompson, Org. Lett., 2011, 13, 1598 CrossRef CAS PubMed
.
- D. M. Baird, R. Hassan and W. K. Kim, Inorg. Chim. Acta, 1987, 130, 39 CrossRef CAS
.
- D. M. Baird and K. Y. Shih, Polyhedron, 1989, 8, 2359 CrossRef CAS
.
- G. E. O. Borgstahl, H. E. Parge, M. J. Hickey, W. F. Beyer Jr, R. A. Hallewell and J. A. Tainer, Cell, 1992, 71, 107 CrossRef CAS
.
- P. Chelikani, I. Fita and P. C. Loewen, Cell. Mol. Life Sci., 2004, 61, 192 CrossRef CAS PubMed
.
- G. Martić, J. T. Engle and C. J. Ziegler, Inorg. Chem. Commun., 2011, 14, 1749 CrossRef PubMed
.
- L. Saussine, E. Brazil, A. Robine, H. Mimoun, J. Fischer and R. Weiss, J. Am. Chem. Soc., 1985, 107, 3534 CrossRef CAS
.
- M. B. Meder, B. A. Siggelkow and L. H. Gade, Z. Anorg. Allg. Chem., 2004, 630, 1962 CrossRef CAS
.
- P. T. Selvi, H. Stoeckli-Evans and M. Palaniandavar, J. Inorg. Biochem., 2005, 99, 2110 CrossRef PubMed
.
- É. Balogh-Hergovich, G. Speier, M. Réglier, M. Giorgi, E. Kuzmann and A. Vértes, Eur. J. Inorg. Chem., 2003, 1735 CrossRef
.
- É. Balogh-Hergovich, G. Speier, B. Tapodi, M. Réglier and M. Giorgi, Z. Kristallogr., 1999, 214, 579 Search PubMed
.
- T. Váradi, J. S. Pap, M. Giorgi, L. Parkanyi, T. Csay, G. Speier and J. Kaizer, Inorg. Chem., 2013, 52, 1559 CrossRef PubMed
.
- J. T. Engle, G. Martić and C. J. Ziegler, Macroheterocycles, 2013, 6, 353 CrossRef CAS
.
- M. Szávuly, R. Csonka, G. Speier, R. Barabás, M. Giorgi and J. Kaizer, J. Mol. Catal. A: Chem., 2014, 392, 120 CrossRef PubMed
.
- M. Szávuly, S. D. Szilvási, R. Csonka, D. Klesitz, G. Speier, M. Giorgi and J. Kaizer, J. Mol. Catal. A: Chem., 2014, 393, 317 CrossRef PubMed
.
- M. Kruck, D. C. Sauer, M. Enders, H. Wadepohl and L. H. Gade, Dalton Trans., 2011, 40, 10406 RSC
.
- D. C. Sauer, M. Kruck, H. Wadepohl, M. Enders and L. H. Gade, Organometallics, 2013, 32, 885 CrossRef CAS
.
- M. A. Robinson, S. I. Trotz and T. J. Hurley, Inorg. Chem., 1967, 6, 392 CrossRef CAS
.
- T. S. Pitchumony and H. Stoeckli-Evans, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2005, 61, 69 Search PubMed
.
- D. N. Marks, W. O. Siegl and R. R. Gagné, Inorg. Chem., 1982, 21, 3140 CrossRef CAS
.
- R. R. Gagné and D. N. Marks, Inorg. Chem., 1984, 23, 65 CrossRef
.
- K.-N. T. Tseng, J. W. Kampf and N. K. Szymczak, Organometallics, 2013, 32, 2046 CrossRef CAS
.
- C. A. Tolman, J. D. Druliner, P. J. Krusic, M. J. Nappa, W. C. Seidel, I. D. Williams and S. D. Ittel, J. Mol. Catal., 1988, 48, 129 CrossRef CAS
.
- B. K. Langlotz, J. L. Fillol, J. H. Gross, H. Wadepohl and L. H. Gade, Chem.–Eur. J., 2008, 14, 10267 CrossRef CAS PubMed
.
- J. A. Camerano, C. Sämann, H. Wadepohl and L. H. Gade, Organometallics, 2011, 30, 379 CrossRef CAS
.
- W. O. Siegl, J. Organomet. Chem., 1976, 107, C27 CrossRef CAS
.
- R. J. Letcher, W. Zhang, C. Bensimon and R. J. Crutchley, Inorg. Chim. Acta, 1993, 210, 183 CrossRef CAS
.
- M. Meder, C. H. Galka and L. H. Gade, Monatsh. Chem., 2005, 136, 1693 CrossRef CAS PubMed
.
- M. Bröring, C. Kleeberg and A. Scheja, Inorg. Chim. Acta, 2011, 374, 572 CrossRef PubMed
.
- M. Bröring and C. Kleeberg, Z. Anorg. Allg. Chem., 2007, 633, 2210 CrossRef
.
- M. Bröring and C. Kleeberg, Z. Anorg. Allg. Chem., 2011, 637, 1769 CrossRef
.
- M. Bröring and C. Kleeberg, Inorg. Chim. Acta, 2007, 360, 3281 CrossRef PubMed
.
- M. Bröring and C. Kleeberg, Chem. Commun., 2008, 2777 RSC
.
- H.-M. Wen, Y.-H. Wu, L.-J. Xu, L.-Y. Zhang, C.-N. Chen and Z.-N. Chen, Dalton Trans., 2011, 40, 6929 RSC
.
- M. B. Meder and L. H. Gade, Eur. J. Inorg. Chem., 2004, 2716 CrossRef CAS
.
- J. Kaizer, J. Pap, G. Speier, M. Réglier and M. Giorgi, Transition Met. Chem., 2004, 29, 630 CrossRef CAS PubMed
.
- D. M. Baird, W. P. Maehlmann, R. D. Bereman and P. Singh, J. Coord. Chem., 1997, 42, 107 CrossRef CAS
.
- T. Csay, B. Kripli, M. Giorgi, J. Kaizer and G. Speier, Inorg. Chem. Commun., 2010, 13, 227 CrossRef CAS PubMed
.
- O. P. Anderson, A. la Cour, A. Dodd, A. D. Garrett and M. Wicholas, Inorg. Chem., 2003, 42, 122 CrossRef CAS PubMed
.
- D. C. Sauer and H. Wadepohl, Polyhedron, 2014, 81, 180 CrossRef CAS PubMed
.
- M. Wicholas, A. D. Garrett, M. Gleaves, A. M. Morris, M. Rehm, O. P. Anderson and A. la Cour, Inorg. Chem., 2006, 45, 5804 CrossRef CAS PubMed
.
- J. L. Cryder, A. J. Killgore, C. Moore, J. A. Golen, A. L. Rheingold and C. J. A. Daley, Dalton Trans., 2010, 39, 10671 RSC
.
- M. Wicholas, B. L. Dietrich, O. P. Anderson and A. la Cour, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2006, 62, m1689 CAS
.
- O. P. Anderson, A. la Cour, A. Berg, A. D. Garrett and M. Wicholas, Inorg. Chem., 2003, 42, 4513 CrossRef CAS PubMed
.
- H. Irving and R. J. P. Williams, J. Chem. Soc., 1953, 3192 RSC
.
- J. Kaizer, R. Csonka, G. Baráth and G. Speier, Transition Met. Chem., 2007, 32, 1047 CrossRef CAS
.
- G. Marongiu and E. C. Lingafelter, Acta Crystallogr., 1982, B38, 620 CrossRef CAS
.
- É. Balogh-Hergovich, G. Speier, M. Réglier, M. Giorgi, E. Kuzmann and A. Vértes, Inorg. Chim. Acta, 2004, 357, 3689 CrossRef PubMed
.
- G. Bullock, A. Cook, A. Foster, L. Rosenberg and L. K. Thompson, Inorg. Chim. Acta, 1985, 103, 207 CrossRef CAS
.
- S. K. Mandal, L. K. Thompson and M. J. Newlands, Inorg. Chim. Acta, 1986, 122, 199 CrossRef CAS
.
- C. L. Sheppard, S. S. Tandon, L. K. Thompson, J. N. Bridson, D. O. Miller, M. Handa and F. Lloret, Inorg. Chim. Acta, 1996, 250, 227 CrossRef CAS
.
- L. K. Thompson, V. Niel, H. Grove, D. O. Miller, M. J. Newlands, P. H. Bird, W. A. Wickramasinghe and A. B. P. Lever, Polyhedron, 2004, 23, 1175 CrossRef CAS PubMed
.
- J. S. Pap, J. Kaizer, M. Giorgi and G. Speier, Inorg. Chem. Commun., 2010, 13, 1069 CrossRef CAS PubMed
.
- M. Albrecht and G. van Koten, Angew. Chem., Int. Ed., 2001, 40, 3750 CrossRef CAS
.
- A. W. Smith, A. Camara-Artigas, M. Wang, J. P. Allen and W. A. Francisco, Biochemistry, 2006, 45, 4378 CrossRef CAS PubMed
.
- L. Prati, M. Rossi and N. Ravasio, J. Mol. Catal., 1992, 75, 347 CrossRef CAS
.
- C. E. Barry, P. G. Nayar and T. P. Begley, Biochemistry, 1989, 28, 6323 CrossRef CAS
.
- K. Anzai, K. Isono, K. Ohkuma and S. Suzuki, J. Antibiot., Ser. A., 1960, 13, 125 CAS
.
- L. I. Simándi, T. M. Barna, L. Korecz and A. Rockenbauer, Tetrahedron Lett., 1993, 34, 717 CrossRef
.
- T. M. Simándi, L. I. Simándi, M. Győr, A. Rockenbauer and Á. Gömöry, Dalton Trans., 2004, 1056 RSC
.
- I. C. Szigyártó, T. M. Simándi, L. I. Simándi, L. Korecz and N. Nagy, J. Mol. Catal. A: Chem., 2006, 251, 270 CrossRef PubMed
.
- C. D. Putnam, A. S. Arvai, Y. Bourne and J. A. Tainer, J. Mol. Biol., 2000, 296, 295 CrossRef CAS PubMed
.
- A. Sigel, H. Sigel and R. K. O. Sigel, Metal Ions in Life Sciences, Neurodegenerative Diseases and Metal Ions, John Wiley & Sons Ltd, 2006, vol. 1 Search PubMed
.
- M. U. Triller, W.-Y. Hsieh, V. L. Pecoraro, A. Rompel and B. Krebs, Inorg. Chem., 2002, 41, 5544 CrossRef CAS PubMed
.
- E. J. Larson and V. L. Pecoraro, J. Am. Chem. Soc., 1991, 113, 7809 CrossRef CAS
.
- A. E. M. Boelrijk and G. C. Dismukes, Inorg. Chem., 2000, 39, 3020 CrossRef CAS
.
- T. Csay, J. Kaizer and G. Speier, React. Kinet. Catal. Lett., 2009, 96, 35 CrossRef CAS PubMed
.
- B. L. Stoddard, P. L. Howell, D. Ringe and G. A. Petsko, Biochemistry, 1990, 29, 8885 CrossRef CAS
.
- L. C. Tabares, J. Gätjens and S. Un, Biochim. Biophys. Acta, 2010, 1804, 308 CrossRef CAS PubMed
.
- J. A. Tainer, E. D. Getzoff, J. S. Richardson and D. C. Richardson, Nature, 1983, 306, 284 CrossRef CAS
.
- D. P. Barondeau, C. J. Kassmann, C. K. Burns, J. A. Tainer and E. D. Getzoff, Biochemistry, 2004, 43, 8038 CrossRef CAS PubMed
.
- J. M. McCord and I. Fridovich, J. Biol. Chem., 1969, 244, 6049 CAS
.
- D. P. Riley, Chem. Rev., 1999, 99, 2573 CrossRef CAS PubMed
.
- E. Suarez-Moreira, J. Yun, C. S. Birch, J. H. H. Williams, A. McCaddon and N. E. Brasch, J. Am. Chem. Soc., 2009, 131, 15078 CrossRef CAS PubMed
.
- B. Ge, F. W. Scheller and F. Lisdat, Biosens. Bioelectron., 2003, 18, 295 CrossRef CAS
.
- W. H. Koppenol, F. Levine, T. L. Hatmaker, J. Epp and J. D. Rush, Arch. Biochem. Biophys., 1986, 251, 594 CrossRef CAS
.
- P. M. Wood, Biochem. J., 1988, 253, 287 CAS
.
- I. Batinic-Haberle, I. Spasojevic, R. D. Stevens, P. Hambright, P. Neta, A. Okado-Matsumoto and I. Fridovich, Dalton Trans., 2004, 1696 RSC
.
- C. Eicken, B. Krebs and J. C. Sacchettini, Curr. Opin. Struct. Biol., 1999, 9, 677 CrossRef CAS
.
- S. J. Smith, C. J. Noble, R. C. Palmer, G. R. Hanson, G. Schenk, L. R. Gahan and M. J. Riley, J. Biol. Inorg. Chem., 2008, 13, 499 CrossRef CAS PubMed
.
- A. J. Skulan, T. C. Brunold, J. Baldwin, L. Saleh, J. M. Bollinger Jr and E. I. Solomon, J. Am. Chem. Soc., 2004, 126, 8842 CrossRef CAS PubMed
.
- M. Srnec, T. A. Rokob, J. K. Schwartz, Y. Kwak, L. Rulisek and E. I. Solomon, Inorg. Chem., 2012, 51, 2806 CrossRef CAS PubMed
.
- J. M. Chitanda, D. E. Prokopchuk, J. W. Quail and S. R. Foley, Dalton Trans., 2008, 6023 RSC
.
- J. M. Chitanda, S.-C. Wu, J. W. Quail and S. R. Foley, Inorg. Chim. Acta, 2011, 379, 122 CrossRef CAS PubMed
.
- E. T. Farinas, C. V. Nguyen and P. K. Mascharak, Inorg. Chim. Acta, 1997, 263, 17 CrossRef CAS
.
- E. W. Y. Wong, J. S. Ovens and D. B. Leznoff, Chem.–Eur. J., 2012, 18, 6781 CrossRef CAS PubMed
.
| This journal is © The Royal Society of Chemistry 2015 |