Assembly of thioether-containing rod-like liquid crystalline materials assisted by hydrogen-bonding terminal carboxyl groups†
Yuki Arakawa,
Sungmin Kang*,
Junji Watanabe and
Gen-ichi Konishi*
Department of Organic and Polymeric Materials, Tokyo Institute of Technology, O-okayama, Meguro-ku, Tokyo 152 8552, Japan. E-mail: konishi.g.aa@m.titech.ac.jp; skang@polymer.titech.ac.jp; Fax: +81-3-5734-2888; Tel: +81-3-5734-2321
First published on 23rd December 2014
Abstract
We designed a hydrogen-bonding tolane-based molecule with terminal carboxyl and alkylsulfanyl groups in an effort to realize thioether-containing rod-like liquid crystalline materials. The molecule successfully exhibits a stable enantiotropic mesophase, in contrast to non-hydrogen bonding derivatives, including tolane and diphenyldiacetylene. In addition, it shows two remarkable characteristics compared to analogues with alkyl or alkoxy groups. First, the mesophase of the alkylsulfanyl derivative shows strong long-range correlation. Second, the birefringence of the alkylsulfanyl derivative is highly temperature-dependent, achieving a maximum value of 0.36. These remarkable characteristics are believed to be due to the high polarisability of sulfur atoms and S–S contacts. These findings will be helpful for the design of novel sulfur-containing rod-like liquid crystalline materials.
Introduction
The development of liquid crystalline (LC) materials containing sulfur atoms as mesogenic moieties has been actively pursued in recent years. These compounds are of interest because of their polarisabilities and van der Waals interactions (comprising dipole interactions due to high polarisabilities and the so-called S–S contacts derived from intra- or intermolecular n–σ* interactions between sulfur atoms), which are greater and stronger, respectively, than their more common analogues containing carbon and oxygen. The latter, in particular, are very important in organic electronics1 as well as the molecular structural chemistry2 of not only LCs but also crystalline materials, because they can lead to well-organized and/or specific molecular assemblies. Therefore, sulfur-containing LC materials including heterocyclic,3 disc-,4 and bent-type5 LC compounds with thioether (alkylsulfanyl) groups (–SR) have been widely reported.However, rod-like (calamitic) LC compounds with alkylsulfanyl groups are quite uncommon. This is because even though sulfur atoms have beneficial effects on birefringence properties and dielectric constants, the rod-like compounds tend to crystallize (i.e. they show no mesophase) or exhibit an unstable monotropic mesophase owing to their strong van der Waals attractions.6 Cross et al. reported the butylsulfanyl group (–SC4H9) as a good candidate for the design of materials with high optical anisotropy; however, no information on stable mesophase was disclosed.6 To the best of our knowledge, there has been no detailed report on the mesophase structures and birefringence properties of rod-like LC materials with alkylsulfanyl groups.
Our recent interests have focused on the design of highly birefringent rod-like LCs and their application as optical materials.3g,7–9 If a nematic phase could be obtained from a thioether-containing rod-like mesogen, an improvement in the birefringence properties of the optical material would be achieved.
To develop novel sulfur-containing rod-like LC materials, we focused on the utilization of hydrogen bonding. Molecules with carboxyl groups are known to generate mesophases owing to the formation of dimers in single-component systems,10 and since the discovery of hydrogen-bonding LC materials in multi-component systems by Kato et al.,11 a large number of studies have been directed at their preparation, characterization, and practical application.4a,12
Herein, we report the synthesis and characterization of a LC phase generated by hydrogen bonding in crystalline rod-like molecules containing an alkylsulfanyl group at the terminal position. Diphenylacetylene compounds with a hexyl (–C6H13; compound 1), hexyloxy (–OC6H13; compound 2), or hexylsulfanyl (–SC6H13; compound 3) chain at one terminus and a carboxyl group at the other were designed and prepared (Chart 1). These molecules, as so-called tolane-based LC materials, successfully formed well-defined enantiotropic mesophases, and their mesophase structures and birefringence (Δn) as an optical property were elucidated and measured, respectively. Interestingly, the alkylsulfanyl derivative exhibited highly correlated long-range mesophases and high birefringence compared with the alkyl and alkoxy derivatives.
 | ||
| Chart 1 Rod-like molecules in this study: tolanes with carboxylic acid (1–3), and other thioether-containing molecules (T1–T6 and DPDA-SC6). | ||
Experimental
Measurements
The 1H NMR and 13C NMR spectra were measured used a JEOL LNM-EX 400 at room temperature. CDCl3 and DMSO-d6 were used as measurement solvents with tetramethylsilane (TMS) as an internal standard. The liquid crystalline textures were investigated by polarizing optical microscopy (POM) (Leica DM2500P microscopy with a Mettler FP90 hot stage) and the transition temperatures and enthalpy changes were measured by differential scanning calorimetry (DSC) (Perkin Elmer DSC7) with heating and cooling scans performed at 10 °C min−1. X-ray investigations were carried out with samples kept in glass capillary tubes (1.5 mm diameter) for oriented patterns under a magnetic field. WAXD measurements were conducted using a Bruker D8 DISCOVER equipped with a Vantec-500 detector using Cu-Kα radiation. The measurements of Δn were performed with a uniaxial aligned nematic cells. The transmittance of light under crossed Nicols conditions was observed as a function of wavelength by a microscope spectroscopic method using a Nikon LV100 Pol optical microscope equipped with a USB4000 (Ocean photonics) spectrometer.Materials
1-Ethynyl-4-hexylbenzene, 4-iodophenol, trimethylsilylacetylene, 4-bromobenzoic acid, 4-bromobenzenethiol, ethynylbenzene, 4-bromobenzonitrile and Pd(PPh3)4 were purchased from TCI, and 6-bromohexane, triethylamine (TEA) and PPh3 were purchased from Wako, and CuI was purchased from Kanto Chemical. Unless otherwise noted, all chemical were commercially available and use as received.Synthetic procedure
After 12 h, insoluble solid was filtrated and the mixture was extracted with ethyl acetate, washed with 2 M HCl aq and brine and dried with MgSO4. After removing the solvent under reduced pressure, the residue was purified with silica gel on column chromatography (eluent: hexane) to afford 3b as colourless liquid. Yield: 79%. 1H NMR (400 MHz, CDCl3) δ 7.35 (d, J = 8.3 Hz, Ar–H, 2H), 7.20 (d, J = 8.3 Hz, Ar–H, 2H), 2.92 (t, J = 7.4 Hz, S–CH2, 2H) 1.64 (tt, J = 7.4 and 7.5 Hz, S–CH2–CH2, 2H), 1.42 (tt, J = 7.1 and 7.5 Hz, S–CH2–CH2–CH2, 2H), 1.33–1.25 (m, CH2–CH2–CH3, 4H), 0.88 (t, J = 7.1 Hz, CH2–CH3, 3H), 0.24 (s, Si–CH3, 9H) ppm.
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH, 1H), 2.93 (t, J = 7.3 Hz, Ar–S–CH2, 2H), 1.65 (tt, J = 7.3 and 7.6 Hz, S–CH2–CH2, 2H), 1.43 (tt, J = 6.9 and 7.6 Hz, S–CH2–CH2–CH2, 2H), 1.34–1.22 (m, CH2–CH2–CH3, 4H), 0.89 (t, J = 7.1 Hz, CH3, 3H) ppm.
CH, 1H), 2.93 (t, J = 7.3 Hz, Ar–S–CH2, 2H), 1.65 (tt, J = 7.3 and 7.6 Hz, S–CH2–CH2, 2H), 1.43 (tt, J = 6.9 and 7.6 Hz, S–CH2–CH2–CH2, 2H), 1.34–1.22 (m, CH2–CH2–CH3, 4H), 0.89 (t, J = 7.1 Hz, CH3, 3H) ppm.Results and discussion
Molecular design
LC materials based on diphenylacetylene are very important, not only for basic LC chemistry but also for various optical applications including liquid crystalline displays (LCDs).13 To explore the effects of sulfur in such materials, we synthesized a variety of sulfur-containing tolane-based compounds, as shown in Chart 1, and investigated their thermal transition behaviours. A symmetrical analogue with hexylsulfanyl groups at the p- and p′-positions (T1: –SC6H13) was prepared, and for comparison, analogues with hexyl (T2: –C6H13) and hexyloxy (T3: –OC6H13) groups were designed to investigate the effects of different tails. The non-substituted analogue (T4: –H) and that with a cyano group (T5: –CN) were prepared to investigate the effects of asymmetry; the latter analogue, especially, is known to form an antiparallel alignment between adjacent molecules in the mesophase.14 The thermal transition behaviours of these compounds were investigated by POM and DSC, and their transition temperatures are shown in Fig. 1. Even though tolane-based materials are known as LC materials with well-defined mesophases, not all of the sulfur-containing compounds exhibited enantiotropic mesophases. Only the hexyl-substituted analogue (T2) showed a monotropic smectic phase during the cooling scan in the POM and DSC measurements, but the LC range was just 13 °C, indicating mesophase instability. We also synthesized a diphenyldiacetylene (DPDA)-type molecule with two hexylsulfanyl groups (DPDA-SC6). This skeleton has a higher aspect ratio than that of tolane, and it is known that such compounds tend to form wide nematic phases owing to their undulating alignment in the mesophases.7,15 However, no mesophases were observed, and only melting and crystallization points were detected for DPDA-SC6, even though it has enough anisotropic structure to generate mesophases.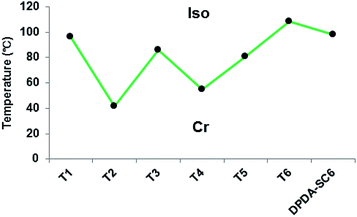 | ||
| Fig. 1 Transition temperatures of various sulfur-containing tolane-based and diphenyldiacetylene molecules. | ||
We attempted to exploit the hydrogen-bonding properties of carboxylic acids to generate stable mesophases, because we conjectured that the disorder and/or flexibility induced by dimerization through hydrogen bonding would allow these compounds to translate and/or rotate to generate mesophases.
Therefore, we designed tolane-based molecules with a hexylsulfanyl group and a carboxylic acid at the p- and p′-positions, respectively. For comparison, analogues with alkyl and alkoxy groups were also synthesized, as shown in Chart 1. A representative synthesis is shown in Scheme 1 for compound 3; the syntheses of compounds 1 and 2 are described in the ESI.† Finally, as can be seen in the representative DSC curve of compound 3 (Fig. 2), we succeeded in generating stable enantiotropic mesophases for a thioether-containing tolane-based molecule, as well as compounds 1 and 2. Clearly, these mesophases are induced by hydrogen bonding, because other crystal tolanes and diphenyldiacetylenes with alkylsulfanyl groups possess enough anisotropy in their structure to exhibit mesophases. Structural analyses of all materials were performed using 1H NMR, 13C NMR, high-resolution mass spectrometry (HRMS), and FT-IR spectroscopy (see ESI†). The broad O–H stretching at 2500–3200 cm−1 in the FT-IR spectra for compounds 1–3 confirms the formation of dimers in the crystal state (Fig. S1†).
POM observations
The phase-transition behaviour of each compound was evaluated by DSC and POM. The transition temperatures and enthalpy changes detected by DSC are listed in Table 1. The DSC curves for compounds 1 and 2 are shown in Fig. S2 and S3,† respectively. As representative textures, the POM images of compounds 2 and 3 obtained using glass plates coated with a planar alignment polyimide, are shown in Fig. 3.| Compound | Transition temperature/°C [enthalpy/kJ mol−1] | Tma | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| a Measured on heating scan.b Highly ordered smectic phase. | ||||||||||
| 1 | Cr1 | 205.8 [3.2] | Cr2 | 213.6 [15.6] | CybC | 219.3 [0.6] | N | 255.8 (5.9) [5.9] | I | 260.2 [5.5] |
| 2 | Cr | 212.3 [19.3] | CybC | 224.8 [0.5] | N | 259.2 [6.3] | I | 259.7 [5.9] | ||
| 3 | Cr | 182.4 [13.1] | SmXb | 188.3 [1.8] | SmC | 222.3 [0.9] | N | 240.7 [5.1] | I | 245.5 [5.5] |
 | ||
| Fig. 3 POM images from in-plane alignment cells. (a) N phase at 230 °C and (b) CybC phase at 220 °C for compound 2, (c) N phase at 200 °C, and (d) SmC phase at 210 °C for compound 3. | ||
For compound 2, a typical nematic (N) phase with marble and schlieren textures is observed on cooling from the isotropic (I) phase at 230 °C (Fig. 3(a)). Upon further cooling to 220 °C, a large number of defect lines appears over the marble texture with no other observable changes, such as the fan-like or broken fan-shaped textural transformations expected for the smectic phase (Fig. 3(b)). This behaviour is also observed for compound 1 (see Fig. S4†). The DSC measurements confirm that these transitions are first-order. These phases are assigned as cybotactic C (CybC) rather than general smectic C (SmC) phases, because they consist of short-range multiple domains of expanded cybotactic C clusters, as determined by XRD measurements (vide infra).
Compound 3 also exhibits a marble texture reminiscent of a typical nematic phase in its high-temperature liquid crystal state (Fig. 3(c)), which is enantiotropic. When cooled to the lower mesophase from the N phase, compound 3 notably shows a general ordered SmC phase with a broken fan-shaped texture (Fig. 3(d)). This result suggests that significant long-range correlation was achieved in this material as a result of the presence of the sulfur atom.
XRD measurements with non-aligned specimens
XRD measurements were performed to reveal the detailed phase structures of the compounds. One- and two-dimensional XRD (1D- and 2D-XRD) patterns of the compounds, obtained using non-aligned specimens, are shown in Fig. 4 and S5–S7,† respectively. From the 1D-XRD patterns of the N phases (Fig. 4(a)), the mean adjacent molecular distances, as determined from the outer broad refractions, are 4.9 Å in compounds 1 and 2, and 4.7 Å in compound 3. The distance for the alkylsulfanyl derivative is the smallest because of the large dipole interactions and S–S contacts acting between adjacent molecules. Furthermore, the formation of dimers can be confirmed from the estimated d-spacings of compounds 1, 2, and 3 corresponding to the small-angle region (27.4, 33.2, and 41.4 Å, respectively), and their calculated molecular lengths (18.0, 19.7, and 20.4 Å, respectively). These results indicate that the mesophases for these molecules are induced by the introduction of the hydrogen-bonding carboxylic acid unit.As shown in Fig. 4(a), the diffraction intensities in the small-angle region are larger than those in the wide-angle region for all the compounds, indicating the formation of cybotactic clusters in the N phases.16 The intensity ratios of the small- and wide-angle peaks (Isax/Iwax) were measured and found to be 1.0 for compound 1 at 240 °C, 1.5 for compound 2 at 254 °C, and 2.4 for compound 3 at 230 °C. It is noteworthy that the alkylsulfanyl derivative exhibits a high ratio, over 2.0, suggesting that it forms larger clusters in the fluid N phase compared with the alkyl and alkoxy derivatives.
The small-angle diffractions of the low-temperature mesophase under the N phases of compounds 1 and 2 are wider than those of typical smectic phases (Fig. 4(b)), suggesting that the domains of compound 1 and 2 have short-range correlations and are dynamic, as the distributions are not Gaussian. The Isax/Iwax ratio is 1.4 for compound 1 at 218 °C and 1.5 for compound 2 at 223 °C; these values are not much higher than those for the corresponding N phases. Hence, these phases are assigned as CybC phases consisting of multiple cybotactic C cluster domains, as reported for bent-type LC molecules.17 Furthermore, there are no striking differences between the 2D-XRD patterns of the CybC phases for compounds 1 and 2 (see Fig. S5 and S6†). Thus, these compounds exhibit disorder induced by hydrogen bonding, and their highly anisotropic molecular structures form dimers, which could not have been formed by their domains in normal smectic layers. In contrast, the diffraction peak of compound 3 is remarkably sharp (Fig. 4(b)), i.e. it corresponds to a conventional smectic phase. At 210 °C, (001) and (002) diffraction peaks are observed at diffraction angles of 2.5 and 5.0 with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 intensity ratio, corresponding to d-spacings of 35.4 and 17.7 Å, respectively. From these results, it is evident that the introduction of the sulfur atom greatly enhances the correlation length in the entire LC phase, including not only the fluid N phase but also the higher-order Sm phase, because its van der Waals attractions are larger than those of carbon and oxygen atoms. Another monotropic transition was detected in the POM images and DSC results under the first typical SmC phase. This transition is characterized as a highly ordered tilted Sm phase (SmX). However, we did not focus on this phase in the current study.
2 intensity ratio, corresponding to d-spacings of 35.4 and 17.7 Å, respectively. From these results, it is evident that the introduction of the sulfur atom greatly enhances the correlation length in the entire LC phase, including not only the fluid N phase but also the higher-order Sm phase, because its van der Waals attractions are larger than those of carbon and oxygen atoms. Another monotropic transition was detected in the POM images and DSC results under the first typical SmC phase. This transition is characterized as a highly ordered tilted Sm phase (SmX). However, we did not focus on this phase in the current study.
XRD measurements using the aligned specimens
XRD measurements were also conducted for specimens oriented by applying a magnetic field to elucidate the phase structures in detail. The 2D-XRD patterns obtained for compounds 2 and 3 are shown in Fig. 5, and the temperature-dependence of the 1D-XRD pattern for each compound is shown in Fig. S8–S10.† The molecular long axis is oriented along the longitudinal direction, as the diffractions in the wide-angle region are along the direction of the magnetic field, which is represented by an arrow. Split diffractions can be observed for all compounds in the small-angle region for the N phase (Fig. 5(a) and (b)), indicating the presence of cybotactic C clusters.18 The splitting maxima of the small-angle scatterings (χ/2) are estimated to be 54° at 230 °C for compound 2 and 45° at 225 °C for compound 1. It is noteworthy that the χ/2 value for compound 3 is 26° at 224 °C, which is approximately half that of the other compounds (Fig. 5(c)). This result can be attributed more to the S–S contacts between sulfur atoms in neighbouring molecules than their dipole interactions, because of the reduction in the angle caused by their proximity. | ||
| Fig. 5 2D-XRD patterns measured for aligned specimens. (a) N phase at 230 °C for compound 2, (b) N phase at 224 °C for compound 3, and (c) SmC phase at 216 °C for compound 3. | ||
We further investigated the dependence of the phase structures of compound 3 on temperature. The obtained diffraction patterns are shown in Fig. S10.† The SmC phase shows a tilt angle of 27° at 212 °C. The angle for the SmC phase is similar to the χ/2 value for the N phase, indicating that, in this case, the cybotactic nematic phase corresponds to a pre-transition state to the smectic C phase. When the temperature of the SmC phase is decreased to 194 °C, the tilt angle from the normal layer is approximately 28°.
From these mesophase structure analyses, we presume that not only the highly anisotropic molecular structures, but also the disorder and/or flexibility arising from hydrogen bonding, allows these crystal molecules to rotate or translate to generate the LC phases. Even the monomeric structures of tolane- and DPDA-based molecules have enough anisotropic structure to generate mesophases. We also confirmed that esterified compound 3 (T6) did not display any mesophases; only a melting point of 108 °C was detected (Chart 1). This result emphasizes the importance of the hydrogen-bonding effect in the generation of mesophases for sulfur-containing rod-like molecules. Compounds 1 and 2 exhibit uncommon disordered phases, in contrast to compound 3, which has long-range correlated mesophases due to its alkylsulfanyl groups. In addition, several reports have indicated that the introduction of hydrogen bonds into a mesogenic structure increases the degree of disorder or flexibility, in contrast to what is seen for LC compounds with mesogens linked by covalent bonds.19
Birefringence measurements
As part of an effort to develop highly birefringent materials and to evaluate the effects of sulfur on the optical properties of these compounds, we measured birefringence (Δn) values in the N phase using microscopic spectroscopy.8 We have previously described this method in detail.9 The typical transmittance data and their fittings, as well as the birefringence of compound 3 at 550 nm and reduced temperatures (Ti − T), are shown in Fig. 6. As can be seen, the Δn values of the compounds can be arranged in the following order: 3 > 2 > 1. In particular, the maximum value obtained for Δn is 0.36 at 227 °C for compound 3. It is noteworthy that the temperature dependence of the birefringence is the greatest for compound 3. The temperature dependence of Δn is given by Δn = Δno(1 − T/Ti)β,20 where Δno is the birefringence for a perfectly oriented material (order parameter; s = 1) and β is a constant that is characteristic of the nematic material. The Δno values approximated by fitting to the above equation reach the extremely high value of 0.80 (β = 0.23) for compound 3, whereas those of compounds 1 and 2 are 0.49 and 0.50, respectively. These results are likely due to the greater polarisability and stronger attractive interactions of the sulfur atoms. Investigations on the dependence of the liquid crystallinity of an analogue on alkyl carbon number are in progress.Conclusion
We designed, synthesized, and characterized a hydrogen-bonded tolane-based LC molecule with terminal carboxyl and alkylsulfanyl groups, and compared it to its alkyl and alkoxy analogues. All three derivatives successfully exhibited enantiotropic mesophases. We concluded that these mesophases were induced by the flexibility and/or disorder derived from the hydrogen bonding of the carboxyl groups, because non-hydrogen bonded rod-like molecules with alkylsulfanyl groups, including tolanes and diphenyldiacetylenes, which possessed sufficient anisotropy in their molecular structures to generate mesophases, did not do so. On analysing the mesophase structures of the prepared compounds, the alkylsulfanyl derivative was found to exhibit larger numbers of highly correlated mesophases than the alkyl and alkoxy derivatives in not only the fluid N phases but also the higher order Sm phases. This is attributed to the larger van der Waals attractions, including dipole interactions and S–S contacts, of the sulfur atoms. In particular, the alkyl and alkoxy derivatives formed highly disordered cybotactic C phases, whereas the alkylsulfanyl derivative exhibited an ordered smectic C phase. In addition, the birefringence of the alkylsulfanyl derivative was enhanced more than those of the alkyl and alkoxy analogues, because of the higher polarisability of the sulfur atom. Moreover, the birefringence was greatly influenced by temperature, with a maximum value of 0.36. We anticipate that comparable behaviour will be observed in other rod-like molecules with hydrogen bonding capability, which would help improve the optical and electronic properties of LC materials.Notes and references
- (a) T. M. Barclay, A. W. Cordes, R. C. Haddon, M. E. Itkis, R. T. Oakley, R. W. Reed and H. Zhang, J. Am. Chem. Soc., 1999, 121, 969 CrossRef CAS; (b) K. Kobayashi, H. Masu, A. Shuto and K. Yamaguchi, Chem. Mater., 2005, 17, 6666 CrossRef CAS; (c) K. Kobayashi, R. Shimaoka, M. Kawahata, M. Yamanaka and K. Yamaguchi, Org. Lett., 2006, 8, 2385 CrossRef CAS PubMed; (d) Y. S. Yang, T. Yasuda, H. Kakizoe, H. Mieno, H. Kino, Y. Tateyama and C. Adachi, Chem. Commun., 2013, 49, 6483 RSC; (e) T. Okamoto, T. Suzuki, H. Tanaka, D. Hashizume and Y. Matsuo, Chem.–Asian J., 2012, 7, 105 CrossRef CAS PubMed; (f) L. Tan, Y. Guo, G. Zhang, Y. Yang, D. Zhang, G. Yu, W. Xu and Y. Liu, J. Mater. Chem., 2011, 21, 18042 RSC; (g) S. Zou, Y. Wang, J. Gao, X. Liu, W. Hao, H. Zhang, H. Zhang, H. Xie, C. Yang, H. Li and W. Hu, J. Mater. Chem. C, 2014, 2, 10011 RSC; (h) Q. Xiao, T. Sakurai, T. Fukino, K. Akaike, Y. Honsho, A. Saeki, S. Seki, K. Kato, M. Takata and T. Aida, J. Am. Chem. Soc., 2013, 135, 18268 CrossRef CAS PubMed.
- (a) Y. Mazaki and K. Kobayashi, J. Chem. Soc., Perkin Trans. 2, 1992, 761 RSC; (b) R. S. Rowland and R. Taylor, J. Phys. Chem., 1996, 100, 7384 CrossRef CAS; (c) J. Dai, M. Munakata, L.-P. Wu, T. Kuroda-Sowa and Y. Suenaga, Inorg. Chim. Acta, 1997, 258, 65 CrossRef CAS; (d) M. Iwaoka, S. Takemoto, M. Okada and S. Tomoda, Bull. Chem. Soc. Jpn., 2002, 75, 1611 CrossRef CAS; (e) Z. García-Hernández, A. Flores-Parra, J. M. Grevy, Á. Ramos-Organillo and R. Contreras, Polyhedron, 2006, 25, 1662 CrossRef PubMed; (f) D. B. Werz, R. Gleiter and F. Rominger, J. Am. Chem. Soc., 2002, 124, 10638 CrossRef CAS PubMed; (g) R. Gleiter and D. B. Werz, Chem. Lett., 2005, 34, 126 CrossRef CAS; (h) G. Mehta, V. Gagliardini, C. Schaefer and R. Gleiter, Org. Lett., 2004, 6, 1617 CrossRef CAS PubMed; (i) M. A. Stokes, R. Kortan, S. R. Amy, H. E. Katz, Y. J. Chabal, C. Kloc and T. Siegrist, J. Mater. Chem., 2007, 17, 3427 RSC.
- (a) A. Seed, Chem. Soc. Rev., 2007, 36, 2046 RSC; (b) M. Funahashi and J. Hanna, Adv. Mater., 2005, 17, 594 CrossRef CAS; (c) A. Matsui, M. Funahashi, T. Tsuji and T. Kato, Chem.–Eur. J., 2010, 16, 13465 CrossRef CAS PubMed; (d) T. Yasuda, T. Shimizu, F. Liu, G. Ungar and T. Kato, J. Am. Chem. Soc., 2011, 133, 13437 CrossRef CAS PubMed; (e) J. I. Tietz, J. R. Mastriana, P. Sampson and A. J. Seed, Liq. Cryst., 2012, 39, 515 CrossRef CAS; (f) S. Kaur, L. Tian, H. Liu, C. Greco, A. Ferrarini, J. Seltmann, M. Lehmann and H. F. Gleeson, J. Mater. Chem. C, 2013, 1, 2416 RSC; (g) Y. Arakawa, S. Kang, S. Nakajima, K. Sakajiri, S. Kawauchi, J. Watanabe and G. Konishi, Liq. Cryst., 2014, 41, 642 CrossRef CAS; (h) S. Kuiper, W. F. Jager, T. J. Dingemans and S. J. Picken, Liq. Cryst., 2009, 36, 389 CrossRef CAS; (i) A. Kovářová, J. Svoboda, V. Novotná, M. Glogarová, M. Salamonczyk, D. Pociecha and E. Gorecka, Liq. Cryst., 2010, 37, 1501 CrossRef; (j) A. Kovářová, M. Kohout, J. Svoboda and V. Novotná, Liq. Cryst., 2014, 41, 1703 CrossRef; (k) J. Herman, O. Chojnowska, P. Harmata, R. W. Dąbrowski, B. Klus and P. Kula, Liq. Cryst., 2014, 41, 1647 CrossRef CAS.
- (a) F. Araoka, S. Masuko, A. Kogure, D. Miyajima, T. Aida and H. Takezoe, Adv. Mater., 2013, 25, 4014 CrossRef CAS PubMed; (b) D. Adam, P. Schumacher, J. Simmerer, L. Haussling, K. Siemensmeyer, K. H. Etzbach, H. Ringsdorf and D. Haarer, Nature, 1994, 371, 141 CrossRef CAS; (c) S. H. Kang, Y.-S. Kang, W.-C. Zin, G. Olbrechts, K. Wostyn, K. Clays, A. Persoons and K. Kim, Chem. Commun., 1999, 1661 RSC; (d) F. Nekelson, H. Monobe, M. Shiro and Y. Shimizu, J. Mater. Chem., 2007, 17, 2607 RSC; (e) S. K. Varshney, H. Nagayama, V. Prasad and H. Takezoe, Liq. Cryst., 2011, 38, 1321 CrossRef CAS; (f) J. Cattle, P. Bao, J. P. Bramble, R. J. Bushby, S. D. Evans, J. E. Lydon and D. J. Tate, Adv. Funct. Mater., 2013, 23, 5997 CrossRef CAS; (g) S. Kumar, Liq. Cryst., 2004, 31, 1037 CrossRef CAS; (h) P. H. J. Kouwer, W. F. Jager, W. J. Mijs and S. J. Picken, J. Mater. Chem., 2003, 13, 458 RSC; (i) P. H. J. Kouwer, W. F. Jager, W. J. Mijs and S. J. Picken, Mol. Cryst. Liq. Cryst., 2004, 411, 1347 CAS.
- (a) I. C. Pintre, J. L. Serrano, M. B. Ros, J. Ortega, I. Alonso, J. M. Perdiguero, C. L. Folcia, J. Etxebarria, F. Goc, D. B. Amabilino, J. P. Luis and E. G. Nadal, Chem. Commun., 2008, 2523 RSC; (b) S. Kang, M. Harada, X. Li, M. Tokita and J. Watanabe, Soft Matter, 2012, 8, 1916 RSC; (c) G. Heppke, D. D. Parghi and H. Sawade, Liq. Cryst., 2000, 27, 313 CrossRef CAS.
- (a) A. J. Seed, K. J. Toyne, J. W. Goodby and D. G. McDonnell, J. Mater. Chem., 1995, 5, 1 RSC; (b) A. J. Seed, K. J. Toyne and J. W. Goodby, J. Mater. Chem., 1995, 5, 2201 RSC; (c) A. J. Seed, K. J. Toyne, M. Hird and J. W. Goodby, Liq. Cryst., 2012, 39, 403 CrossRef CAS; (d) G. J. Cross, A. J. Seed, K. J. Toyne, J. W. Goodby, M. Hird and M. C. Artal, J. Mater. Chem., 2000, 10, 1555 RSC.
- (a) Y. Arakawa, S. Nakajima, S. Kang, G. Konishi and J. Watanabe, J. Mater. Chem., 2012, 22, 14346 RSC; (b) Y. Arakawa, S. Kang, S. Nakajima, K. Sakajiri, Y. Cho, S. Kawauchi, J. Watanabe and G. Konishi, J. Mater. Chem. C, 2013, 1, 8094 RSC.
- (a) Y. Arakawa, S. Nakajima, S. Kang, M. Shigeta, G. Konishi and J. Watanabe, J. Mater. Chem., 2012, 22, 13908 RSC; (b) S. Kang, S. Nakajima, Y. Arakawa, M. Tokita, J. Watanabe and G. Konishi, Polym. Chem., 2014, 5, 2253 RSC; (c) Y. Arakawa, S. Nakajima, S. Kang, G. Konishi and J. Watanabe, Liq. Cryst., 2012, 39, 1063 CrossRef CAS; (d) Y. Arakawa, S. Kang, J. Watanabe and G. Konishi, Chem. Lett., 2014, 43, 1858 CrossRef CAS.
- (a) Y. Arakawa, S. Nakajima, R. Ishige, M. Uchimura, S. Kang, G. Konishi and J. Watanabe, J. Mater. Chem., 2012, 22, 8394 RSC; (b) S. Kang, S. Nakajima, Y. Arakawa, G. Konishi and J. Watanabe, J. Mater. Chem. C, 2013, 1, 4222 RSC; (c) M. Uchimura, S. Kang, R. Ishige, J. Watanabe and G. Konishi, Chem. Lett., 2010, 39, 513 CrossRef CAS.
- (a) A. E. Bradfield and B. Jones, J. Chem. Soc., 1929, 2660 RSC; (b) G. W. Gray and B. Jones, J. Chem. Soc., 1953, 4179 CAS; (c) A. J. Herbert, Trans. Faraday Soc., 1967, 63, 555 RSC; (d) C. Kavitha, N. P. S. Prabu and M. L. N. M. Mohan, Mol. Cryst. Liq. Cryst., 2014, 592, 163 CrossRef CAS; (e) M. Roohnikan, M. Ebrahimi, S. R. Ghaffarian and N. Tamaoki, Liq. Cryst., 2013, 40, 314 CrossRef CAS; (f) T. Iwata, R. Miyata and A. Matsumoto, Chem. Lett., 2013, 42, 849 CrossRef CAS.
- (a) T. Kato and J. M. J. Frechet, J. Am. Chem. Soc., 1989, 111, 8533 CrossRef CAS; (b) T. Kato, T. Uryu, F. Kaneuchi, C. Jin and J. M. J. Fréchet, Liq. Cryst., 1993, 14, 1311 CrossRef CAS; (c) T. Kato, N. Mizushita and K. Kishimoto, Angew. Chem., Int. Ed., 2005, 45, 38 CrossRef PubMed.
- (a) F. Vera, R. M. Tejedor, P. Romero, J. Barber, M. B. Ros, J. L. Serrano and T. Sierra, Angew. Chem., Int. Ed., 2007, 46, 1873 CrossRef CAS PubMed; (b) K. Kishikawa, S. Nakahara, Y. Nishikawa, S. Kohmoto and M. Yamamoto, J. Am. Chem. Soc., 2005, 127, 2565 CrossRef CAS PubMed; (c) J. Del Barrio, E. Blasco, C. Toprakcioglu, A. Koutsioubas, O. A. Scherman, L. Oriol and C. Sanchez-Somolinos, Macromolecules, 2014, 47, 897 CrossRef CAS; (d) M. Amorín, A. Pérez, J. Barberá, H. L. Ozores, J. L. Serrano, J. R. Granja and T. Sierra, Chem. Commun., 2014, 688 RSC; (e) T. Kato and K. Tanabe, Chem. Lett., 2009, 38, 634 CrossRef CAS; (f) A. G. Cook, A. Martinez-Felipe, N. J. Brooks, J. M. Seddon and C. T. Imrie, Liq. Cryst., 2013, 40, 1817 CrossRef CAS; (g) D. Stewart and C. T. Imrie, J. Mater. Chem., 1995, 5, 223 RSC; (h) C. M. Paleos and D. Tsiourvas, Liq. Cryst., 2001, 28, 1127 CrossRef CAS; (i) K. Kishikawa, M. Isaka, M. Takahashi, K. Saito and S. Kohmoto, Chem. Lett., 2011, 40, 1278 CrossRef CAS; (j) M. D. Miranda, F. V. Chávez, T. M. R. Maria, M. E. S. Eusebio, P. J. Sebastião and M. R. Silva, Liq. Cryst., 2014, 41, 1743 CrossRef CAS.
- (a) G. W. Skelton, D. Dong, R. P. Tuffin and S. M. Kelly, J. Mater. Chem., 2003, 13, 450 RSC; (b) S. Gauza, H. Wang, C. H. Wen, S. T. Wu, A. J. Seed and R. Dabrowski, Jpn. J. Appl. Phys., 2003, 42, 3463 CrossRef CAS; (c) S. Gauza, X. Zhu, W. Piecek, R. Dabrowski and S.-T. Wu, J. Disp. Technol., 2007, 3, 250 CrossRef CAS; (d) Q. Song, S. Gauza, H. Xianyu, S. T. Wu, Y. M. Liao, C. Y. Chang and C. S. Hsu, Liq. Cryst., 2010, 37, 139 CrossRef CAS; (e) O. Catanescua and L. C. Chien, Liq. Cryst., 2006, 33, 115 CrossRef; (f) J. Hermana, J. Dziaduszek, R. Dąbrowski, J. Kędzierski, K. Kowiorski, V. S. Dasari, S. Dhara and P. Kula, Liq. Cryst., 2013, 40, 1174 CrossRef; (g) J. Dziaduszek, P. Kula, R. Dąbrowski, W. Drzewiński, K. Garbat, S. Urban and S. Gauza, Liq. Cryst., 2014, 39, 239 CrossRef; (h) B. Li, W. He, L. Wang, X. Xiao and H. Yang, Soft Matter, 2013, 9, 1172 RSC; (i) S.-J. Wang, R.-Y. Zhao, S. Yang, Z.-Q. Yu and E.-Q. Chen, Chem. Commun., 2014, 8378 RSC; (j) E. Levert, S. Lacelle, E. Zysman-Colman and A. Soldera, J. Mol. Liq., 2013, 183, 59 CrossRef CAS PubMed; (k) M. Hu, Z. An, J. Li, L. Mo, Z. Yang, J. Li, Z. Che and X. Yang, Liq. Cryst., 2014, 41, 1696 CrossRef CAS; (l) D. V. Sai, P. Sathyanarayana, V. S. S. Sastry, J. Herman, P. Kula, R. Dabrowski and S. Dhara, Liq. Cryst., 2014, 41, 591 CrossRef.
- (a) A. J. Leadbetter, R. M. Richardson and C. N. Colling, J. Phys., 1975, 3, C1–C37 Search PubMed; (b) J. E. Lydon and C. J. Coakley, J. Phys., 1975, 3, C1–C45 Search PubMed; (c) C. Amovilli, I. Cacelli, S. Campanile and G. Prampolini, J. Chem. Phys., 2002, 117, 15 CrossRef PubMed.
- L. Zhu, H. Tran, F. L. Beyer, S. D. Walck, X. Li, H. Ågren, K. L. Killops and L. M. Campos, J. Am. Chem. Soc., 2014, 136, 13381 CrossRef CAS PubMed.
- A. de Vries, J. Mol. Liq., 1986, 31, 193 CrossRef CAS.
- C. Keith, A. Lehmann, U. Baumeister, M. Prehma and C. Tschierske, Soft Matter, 2010, 6, 1704 RSC.
- A. De Vries, Mol. Cryst. Liq. Cryst., 1970, 10, 219 CrossRef CAS.
- (a) E. Barmatov, S. Grande, A. Filippov, M. Barmatova, F. Kremer and V. Shibaev, Macromol. Chem. Phys., 2000, 201, 2603 CrossRef CAS; (b) Y. Uchida, K. Suzuki and R. Tamura, J. Phys. Chem. B, 2012, 116, 9791 CrossRef CAS PubMed; (c) W. He, G. Pan, Z. Yang, D. Zhao, G. Niu, W. Huang, X. Yuan, J. Guo, H. Cao and H. Yang, Adv. Mater., 2009, 21, 2050 CrossRef CAS.
- I. Haller, Prog. Solid State Chem., 1975, 10, 103 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section: synthesis and identification, POM imaging, DSC, XRD, and evaluation of birefringence. See DOI: 10.1039/c4ra15300f |
| This journal is © The Royal Society of Chemistry 2015 |




