Application of nanoparticle mediated N-arylation of amines for the synthesis of pharmaceutical entities using vit-E analogues as amphiphiles in water†
Atul Kumar* and
Ajay Kumar Bishnoi
Medicinal and Process Chemistry Division, CSIR-Central Drug Research Institute, Lucknow, 226031, India. E-mail: dratulsax@gmail.com; Fax: +91-522-26234051; Tel: +91-522-2612411
First published on 4th February 2015
Abstract
The first CuI-nanoparticle catalyzed inter and intramolecular N-arylation of amines using vitamin E analogues (TPGS) as amphipiles has been developed in water. Application of this transition metal–amphiphile C–N bond formation methodology is further extended for the synthesis of substituted indoles, bioactive natural product tryptanthrin and intermediates of pharmaceutical entities such as imatinib, nilotinib, selective D3 agonist/antagonist ligands, and oxacarbazepine.
Introduction
In the twenty first century chemical transformations are not only evaluated by yield of a synthesis/process, but a complete art of synthesis is required for the evaluation of a synthesis/process. The formula for evaluation of synthesis becomes YES (Yield, Economics – eco friendliness and Stereochemistry).1 To address economics and environmental sustainability emphasis is direct towards development of green medium, and recyclable reagents.2Development of green medium is fascinating area since the ionic liquid has been introduced but later on IL biodegradability and greenness in under question mark.3 Therefore water is consider as the best available choice in nature. Water a solvent of life, as almost all the biochemical reaction under goes in water.4 However, poor aqueous solubility of organic compounds or reactivity of many transition-metal/organo catalysts are the main limitation, which can be overcome by the introducing organic microenvironment in aqueous phase.5
A smart strategy to solve these issues is micellar catalysis.6 Recently developed the transition metal coupling reaction catalyzed in water with TPGS-750-M/PTS.7 TPGS-750-M is a biodegradable and water-soluble derivative of natural vitamin E, which is formed by esterification of vitamin E succinate with polyethylene glycol (PEG) 1000.8 It is consisting of a tocopherol (vitamin E) hydrophobic group and a PEG hydrophilic group. Micellar catalysis greenness methodology which allows the E factor approach to zero.9 Micelles are mainly simple spherical supramolecules, which are formed by amphiphiles in water and have ability to solubilization of both nonpolar and polar substrates, reagents, and catalysts.10
 | ||
| Fig. 1 N-Aryaltion of amines reaction using vit-E amphiphile in water and their position on HLB scale. | ||
Lipshutz et al. reported N-aryaltion of amines reaction using PTS as nonionic amphiphile with [(p-allyl)PdCl]2 as catalyst, Takasago's as ligand and base in presence of water.11 Inspired by this report we wish to report here first CuI-nanoparticle catalyzed inter and intramolecular N-arylation of amines using vitamin E analogues (TPGS) as amphipile in water (Fig. 1). To the best of our knowledge this is first report on Cu nano and vitamin E analogues as amphiphile used for catalyzing C–N bond formation. We utilised this methodology for the synthesis of medicinally important indoles,12 anticancer and antitubercular natural product tryptanthrin.13 We further extended the protocol for the synthesis of intermediates of pharmaceutical entities such as clinically used anticancer drugs14 imatinib, nilotinib, selective D3 agonist/antagonist ligand15 for the treatment of alzhemer's and, drug addiction therapy and anticonvulsant oxacarbazepine.16
Our initial efforts were focused on the actual effectiveness of an efficient nano catalyst for the N-arylation of amines reaction in nanomicelles in water. For this investigation the reaction of bromobenzene and morpholine, taken as a model reaction, control experiment carried out “on water” (i.e., without amphiphile) led to no product formation after 10 h at room temperature as well as refluxing.
We further carried out the reaction with nano CuI catalyst at room temperature in water for 10 h but found very less yield. After that we was explore nonionic amphiphile such as oil/water emulsifier and detergent. However, the use of amphiphile (Brij 30) and nano CuI resulted in the formation of the desired product in 65% yield at room temperature. In search of an efficient amphiphile, we further screened various amphiphiles in the model N-arylation of amines reaction in water such as Brij 30, Triton X-100 and PTS. Therefore, TPGS was found to be the best amphiphile with nano CuI for the synthesis of N-arylation of amines in water.
The copper(I) salts such as CuBr, CuCl, Cu2O, and CuI were found to be inferior to CuI nanoparticles (Table 1, entries 11–15). Among the bases studied, Et3N, K2CO3, KOtBu, K3PO4, provided lower yields than NaOH (Table 1, entries 1–5). Of the copper catalysts investigated, CuI nanoparticles were significantly superior to other catalyst (Table 1, entries 1). In order to evaluate the catalyst loading, the reaction was carried out using 1.5, 3.5, and 5.0% mol nano CuI at room temperature in water. It was found that 3.5% mol NP-CuI is sufficient to give the desired product in excellent yield with an enhanced rate (Table 1, entries 8–10).
| Entry | [Cu] (mol%) | Base | Solvent | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: bromobenzene (1.0 mmol), morpholine (1.5 mmol), CuI-NP (3.5% mol), NaOH as base (3 equ.), aqueous TPGS (2 wt%) amphiphile solution (5 ml) as solvent for 4.5 h at rt.b Isolated yield. np = nanoparticles, TPGS (DL-α-tocopherol methoxypolyethylene glycol succinate), PTS (polyoxyethanyl-α-tocopheryl sebacate). | ||||
| 1 | CuI (np (3.5)) | NaOH | TPGS | 92 |
| 2 | CuI (np (3.5)) | KOtBu | TPGS | 80 |
| 3 | CuI (np (3.5)) | K2CO3 | TPGS | 72 |
| 4 | CuI (np (3.5)) | Et3N | TPGS | 65 |
| 5 | CuI (np (3.5)) | K3PO4 | TPGS | 52 |
| 6 | CuI (np (3.5)) | NaOH | Brij 30 | 65 |
| 7 | CuI (np (3.5)) | NaOH | Triton X-100 | 72 |
| 8 | CuI (np (3.5)) | NaOH | PTS | 80 |
| 9 | CuI (np (1.5)) | NaOH | TPGS | 78 |
| 10 | CuI (np (5.0)) | NaOH | TPGS | 90 |
| 11 | CuI (15) | NaOH | TPGS | 70 |
| 12 | Cu2O | NaOH | TPGS | 48 |
| 13 | Cu(OAc)2 | NaOH | TPGS | 52 |
| 14 | CuBr | NaOH | TPGS | 48 |
| 15 | CuCl | NaOH | TPGS | 35 |
A control reaction carried out “on water” (i.e., in the absence of amphiphile) confirmed the importance of micellar catalysis in facilitating N-arylation of amines coupling reaction in aqueous media (Table 2).
| Entry | Conc.(wt%) | Amphiphile | Yield (%) |
|---|---|---|---|
| 1 | 2% | TPGS | 92 |
| 2 | 1% | TPGS | 70 |
| 3 | 5% | TPGS | 82 |
| 4 | 0 | TPGS | 10 |
A lower concentration of amphiphile was therefore deemed favorable, although too low a concentration or its complete absence was detrimental. Consequently, TPGS (2 wt%) was selected for further studies. A wide array of aromatic amines, azoles, secondary amines were under goes N-arylation with aryl halides in excellent yields (Scheme 1).
To explore this methodology, we have economically synthesized the intermediate of high profile anticancer drugs such as imatinib, nilotinib. The process exhibits a broad scope for the synthesis of intermediates of imatinib, nilotinib, selective D3 receptor ligand, bioactive natural product tryptanthrin, substituted indoles, and oxacarbazepine respectively (Schemes 2–7). After completion of reaction, the catalyst was recovered from the reaction mixture by centrifugation and reused for the next fresh reaction. It is noteworthy that the catalyst could be reused at least five times without any significantly loss of efficiency. Therefore, we suspected that because of large surface area, nanoparticles have very high catalytic properties as compare to other catalyst (Table 3).
Application of intermolecular N-aryl amination
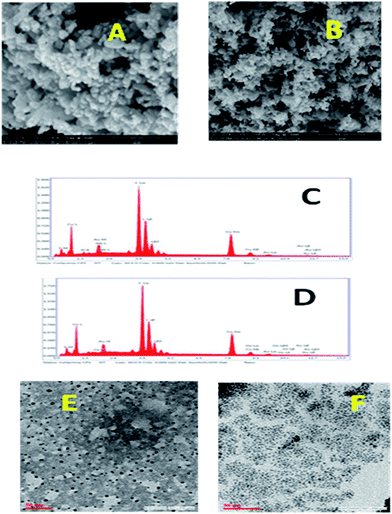
The surface property and the composition of the catalyst were characterized from scanning electron microscope (SEM), transmission electron microscope (TEM) and energy dispersive X-ray analysis (EDX). The EDX spectrum further authenticates the presence of Cu in the nanocomposite. In addition, in the SEM, TEM analysis of CuI nanoparticles, interestingly, the shape and size of the nanoparticles remained unchanged before and after the reaction (shown in Fig. 2).
Conclusions
In summary, we have developed first CuI-nanoparticle catalyzed inter and intramolecular N-arylation of amines using vitamin E analogues (TPGS) as amphiphile in aqueous environment. This methodology is further extended to bioactive natural product tryptanthrin, intermediates of pharmaceutical entity such as life saving anticancer drugs imatinib, nilotinib and anticonvulsant drug oxacarbazepine. The amphiphile used is biodegradable and catalyst have good recyclability. The nano CuI mediated organic synthesis (NAMO-synthesis) in aqueous media via micellar catalysis has immense future in application in the area of medicinal chemistry and material science.| Run | Catalyst recovery (%) | Product yield (%) |
|---|---|---|
| a CuI nanoparticles (3.5 mol%), bromobenzene (1.0 mmol), morpholine (1.5 mmol) base (1.5 equ.), aqueous TPGS (2 wt%) amphiphile solution (5 ml) as solvent for 4.5 h at rt (room temperature).b The recovered catalyst was used under identical reaction conditions to those for the first run. | ||
| 1a | 85 | 92 |
| 2b | 81 | 86 |
| 3b | 74 | 82 |
| 4b | 72 | 80 |
| 5b | 70 | 75 |
Experimental section
General procedure for preparation of CuI nanoparticles
0.464 g (4 mmol) of dimethylglyoxime (dmgH) and 0.400 g (2 mmol) of Cu(OAc)2·H2O were added into 50 ml of absolute ethanol in sequence, which was stirred at 0 °C for 30 min to get brown precipitates Cu(dmg)2. Then the collected precipitates dispersed in 50 ml of absolute ethanol again, 0.664 g (4 mmol) KI was added and stirred vigorously for 2 h. After that, the mixture was transferred into 60 ml Teflon-lined stainless steel autoclave. The autoclave was sealed and heated at 180 °C for 6 h, and then the reactor bomb is allowed to cool to room temperature. Black precipitates were obtained, then centrifugalized and washed with ethanol and deionized water for three times to ensure the removal of the impurities. The final product was then dried in a vacuum oven at room temperature for 12 h.17General procedure for the N-arylation of amine/azoles
The N-arylation of amines was carried out in a round bottomed flask. In a typical experiment, a mixture of chlorobenzene (1 mmol), morpholine (1.5 mmol), CuI NPs (3.5 mol%) and NaOH (3 equ.) were dissolved in aqueous TPGS (2 wt%) amphiphile solution (5 ml) as solvent and stirred for the 4.5 hours at room temperature. The reaction was monitored to completion using TLC. At the end of reaction, the mixture was then cooled to room temperature and poured into distilled water. The products were extracted using EtOAc and the organic layer was dried over anhydrous sodium sulphate (Na2SO4). The solvent was evaporated in vacuo, the crude products were purified by column chromatography using EtOAc/hexane solvent system.General procedure for the 2-bromoindole derivatives in one-pot
The intramolecular amination reaction was carried out in a round bottomed flask. In a typical experiment, a mixture of 2-(2,2-dibromovinyl)aniline (1 mmol), CuI NPs (3.5 mol%) and NaOH (3.0 equ.) were dissolved in aqueous TPGS (2 wt%) amphiphile solution (5 ml) as solvent and stirred for the 15 hours at 90 °C temperature. The reaction was monitored to completion using TLC. At the end of reaction, the mixture was then cooled to room temperature and poured into distilled water. The products were extracted using EtOAc and the organic layer was dried over anhydrous sodium sulphate (Na2SO4). The solvent was evaporated in vacuo, the crude products were purified by silica column chromatography using EtOAc/hexane solvent system.Acknowledgements
A. K. B. is thankful to CSIR-SRF New Delhi for financial support. The authors also acknowledge analytical facility of CSIR-CDRI and Dr Rajnish K. Chaturvedi, IITR, for SEM and TEM. Financial support from CSIR-CDIR network project BSC-0104.CDRI communication no: 8915.Notes and references
- (a) S. Kobayashi, Adv. Synth. Catal., 2002, 344, 219 CrossRef CAS; (b) S. Kobayashi and K. Manabe, Acc. Chem. Res., 2002, 35, 209–217 CrossRef CAS PubMed; (c) R. Ciriminna and M. Pagliaro, Org. Process Res. Dev., 2013, 17, 1479–1484 CrossRef CAS; (d) U. M. Lindstrom, Chem. Rev., 2002, 102, 2751 CrossRef PubMed; (e) C. J. Li, Chem. Rev., 2005, 105, 3095 CrossRef CAS PubMed; (f) D. J. C. Constable, A. D. Curzons and V. L. Cunningham, Green Chem., 2002, 4, 521–527 RSC.
- (a) M. E. Zakrzewska, E. B. Łukasik and R. B. Łukasik, Chem. Rev., 2011, 111, 397–417 CrossRef CAS PubMed; (b) N. Azizi, E. Batebi, S. Bagherpour and H. Ghafuri, RSC Adv., 2012, 2, 2289–2293 RSC; (c) D. G. Blackmond, A. Armstrong, V. Coombe and A. Wells, Angew. Chem., Int. Ed., 2007, 46, 3798–3800 (Angew. Chem., Int. Ed., 2007, 46, 3798–3800) CrossRef CAS PubMed; (d) A. Kumar and A. K. Bishnoi, RSC Adv., 2014, 4, 31973–31976 RSC.
- (a) A. Romero, A. Santos, J. Tojo and A. Rodríguez, J. Hazard. Mater., 2008, 151, 268–273 CrossRef CAS PubMed; (b) N. V. Plechkova and K. R. Seddon, Chem. Soc. Rev., 2008, 37, 123–150 RSC; (c) A. Kumar and A. K. Bishnoi, RSC Adv., 2014, 4, 41631–41635 RSC.
- (a) P. A. Grieco, Organic Synthesis in Water, Blacky Academic and Professional, London, 1998 Search PubMed; (b) C. J. Li and T. H. Chan, Organic Reactions in Aqueous Media, John Wiley & Sons, New York, 1997 Search PubMed; (c) B. Cornils and W. A. Herrmann, Aqueous- Phase Organometallic Chemistry: Concepts and Applications, Wiley-VCH, Weinheim, 1998 Search PubMed.
- (a) T. Tang, T. Metanawin, A. Hebden, P. McGowan and X. S. Wang, Chem. Commun., 2010, 46, 6663–6665 RSC; (b) N. S. Ieong, K. Brebis, L. E. Daniel, R. K. O'Reilly and M. I. Gibson, Chem. Commun., 2011, 47, 11627–11629 RSC; (c) A. Xie, M. Cao, Y. Liu, L. Feng, X. Hu and W. Dong, Eur. J. Org. Chem., 2014, 436–441 CrossRef CAS.
- (a) T. Dwars, E. Paetzold and G. Oehme, Angew. Chem., 2005, 117, 7338–7364 (Angew. Chem., Int. Ed., 2005, 44, 7174–7199) CrossRef; (b) B. H. Lipshutz, D. W. Chung and B. Rich, Org. Lett., 2008, 10, 3793–3796 CrossRef CAS PubMed; (c) B. H. Lipshutz and A. R. Abela, Org. Lett., 2008, 10, 5329–5332 CrossRef CAS PubMed.
- (a) B. H. Lipshutz and S. Ghorai, Aldrichimica Acta, 2008, 41, 59–72 (Aldrichimica Acta, 2012, 45, 3–16) CAS; (b) H. Borowy-Borowski, M. Sikorska-Walker and P. R. Walker, U.S. Patent 6,045,826, Apr 4, 2000PatenttransU.S. Patent 6,191,172, Feb 20, 2001PatenttransU.S. Patent 6,632,443, Oct 14, 2003; (c) B. H. Lipshutz, N. A. Isley, J. C. Fennewald and E. D. Slack, Angew. Chem., Int. Ed., 2013, 52, 10952–10958 CrossRef CAS PubMed.
- B. H. Lipshutz, S. Ghorai, A. R. Abela, R. Moser, T. Nishikata, C. Duplais and A. Krasovskiy, J. Org. Chem., 2011, 76, 4379–4391 CrossRef CAS PubMed.
- (a) N. Miyaura and A. Suzuki, Chem. Rev., 1995, 95, 2457 CrossRef CAS; (b) B. H. Lipshutz, N. A. Isley, J. C. Fennewald and E. D. Slack, Angew. Chem., Int. Ed., 2013, 52, 10592 Search PubMed.
- (a) T. Dwars, E. Paetzold and G. Oehme, Angew. Chem., 2005, 117, 7338–7364 (Angew. Chem., Int. Ed., 2005, 44, 7174–7199) CrossRef; (b) N. Giri and S. L. James, Chem. Commun., 2011, 47, 245–247 RSC; (c) S. R. K. Minkler, B. H. Lipshutz and N. Krause, Angew. Chem., Int. Ed., 2011, 50, 7820–7823 CrossRef CAS PubMed.
- B. H. Lipshutz, D. W. Chung and B. Rich, Adv. Synth. Catal., 2009, 351, 1717–1721 CrossRef CAS PubMed.
- (a) N. K. Kaushik, N. Kaushik, P. Attri, N. Kumar, C. H. Kim, A. K. Verma and E. H. Choi, Molecules, 2013, 18, 6620 CrossRef CAS PubMed; (b) H. Shirinzadeh, B. Eren, H. G. Orhan, S. Suzen and S. Ozden, Molecules, 2010, 15, 2187 CrossRef CAS PubMed.
- S. Yang, X. Li, F. Hu, Y. Li, Y. Yang, J. Yan, C. Kuang and Q. Yang, J. Med. Chem., 2013, 56, 8321–8331 CrossRef CAS PubMed.
- (a) F. Stegmeier, M. Warmuth, W. R. Sellers and M. Dorsch, Clin. Pharmacol. Ther., 2010, 87, 543–552 CrossRef CAS PubMed; (b) H. Kantarjian, et al., N. Engl. J. Med., 2006, 354, 2542–2551 CrossRef PubMed.
- C. Fischer and B. Koenig, Beilstein J. Org. Chem., 2011, 7, 59–74 CrossRef CAS PubMed.
- M. Mazza, G. D. Marca, M. D. Nicola, G. Martinotti, G. Pozzi, L. Janiri, P. Bria and S. Mazza, Epilepsy Behav., 2007, 10, 397–401 CrossRef PubMed.
- (a) X. L. Li, X. Y. Zhu, T. L. Duan and Y. T. Qian, Solid State Commun., 2006, 138, 526–529 CrossRef CAS PubMed; (b) H. J. Xu, Y. F. Liang, X. F. Zhou and Y. S. Feng, Org. Biomol. Chem., 2012, 10, 2562–2568 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and NMR spectra. See DOI: 10.1039/c4ra15267k |
| This journal is © The Royal Society of Chemistry 2015 |