Revealing the properties of the cubic ZrO2 (111) surface by periodic DFT calculations: reducibility and stabilization through doping with aliovalent Y2O3
Chiara Riccaa,
Armelle Ringuedéa,
Michel Cassira,
Carlo Adamoab and
Frédéric Labat*a
aPSL Research University, Chimie Paristech-CNRS, Institut de Recherche de Chimie de Paris, 11 rue Pierre et Marie Curie, F-75231 Paris Cedex 05, France. E-mail: frederic.labat@chimie-paristech.fr
bInstitut Universitaire de France, 103 Bd Saint-Michel, F-75005, Paris, France
First published on 20th January 2015
Abstract
A detailed theoretical study concerning the formation of oxygen vacancies on the clean (111) surface of cubic ZrO2 and the structural and electronic properties of the (111) surface of yttria-stabilized zirconia (YSZ, 8 mol% Y2O3) was carried out using DFT methods in a periodic approach. For the formation of oxygen defects on the clean (111) surface, two different oxygen vacancy positions and two possible spin states for each position were investigated. Large vacancy formation energy, small relaxation and the presence of a highly localized state in the gap characterize the formation of oxygen defects on this surface. Regarding the yttria-stabilized surface, a systematic study of the stability, geometry and electronic structure of seven different configurations for Y atoms and oxygen vacancies on the surface was performed. The doping with Y2O3 stabilizes the cubic (111) ZrO2 surface and is accompanied by large relaxations of the O atoms NN to the vacancies. In addition, Y atoms preferentially occupy positions NNN to the defect. Despite the presence of  vacancies in YSZ, no mid-gap states have been observed in any of the studied arrangements. This study allowed identifying an accurate computational protocol and a suitable model of the (111) surface of YSZ, through the characterization of its structural and electronic properties. Both could be used to further elucidate the role of YSZ as electrolyte in SOFC applications, with a view to better clarifying the basic operating principles of low temperature solid oxide fuel cells (LT-SOFCs).
vacancies in YSZ, no mid-gap states have been observed in any of the studied arrangements. This study allowed identifying an accurate computational protocol and a suitable model of the (111) surface of YSZ, through the characterization of its structural and electronic properties. Both could be used to further elucidate the role of YSZ as electrolyte in SOFC applications, with a view to better clarifying the basic operating principles of low temperature solid oxide fuel cells (LT-SOFCs).
I. Introduction
Solid oxide fuel cells (SOFCs) have recently attracted a lot of attention because of their high-energy efficiency, fuel flexibility and low pollutant emissions, which place them as one of the most promising and environmentally friendly in the field of energy conversion devices.1 Yttria-stabilized zirconia (YSZ) is the most widely used electrolyte for conventional SOFCs operating at high temperature (>800 °C), where YSZ shows high ionic conductivity. However, as a consequence of the drawbacks associated to these operating conditions (such as material degradation, technological complications and high running costs), the current SOFC research is oriented toward the development of novel electrolytes that allow working conditions with lower temperatures (LT-SOFCs). In this regard, composite materials with enhanced conductivity based on a molten salt and an oxide have been recently proposed as promising electrolytes for LT-SOFCs (LT, <600 °C).2 Ceria is generally associated with molten carbonates for these composite compounds,3–5 but the use of YSZ, for instance, could also be envisaged.6A deeper understanding of the structural and electronic properties of these materials constitutes always the first step toward the comprehension of their role as electrolyte in these devices.
Pure zirconia (ZrO2) exhibits three main crystallographic structures stable at ambient pressure: the monoclinic, tetragonal, and cubic polymorphs. At room temperature, zirconia adopts the monoclinic baddeleyite structure (P21/c), stable up to 1480 K where it transforms into the tetragonal polymorph (P42/nmc). At around 2650 K, the tetragonal ZrO2 converts into a cubic fluorite structure (Fm3m).7 It has been observed that Zr4+ ions appear to be too large for an efficiently packed rutile structure and at the same time too small to form the eight-fold coordinated fluorite structure.8,9 For these reasons, during cooling, the high temperature cubic structure is not stable and distorts first into the lower symmetry tetragonal phases (where Zr cations are still eight-fold coordinated) and finally into the lowest symmetry monoclinic phase (which has seven coordinated Zr cations).
Nonetheless, tetragonal and cubic phases can be stabilized at lower temperatures through doping with aliovalent oxides like yttria (Y2O3).7 For each pair of aliovalent Y3+ ions that substitutes a pair of Zr4+ cations, one oxygen vacancy must be created to ensure the neutrality of the system.
The first effect of the doping is the partial or complete structural stabilization, depending on the dopant concentration. At room temperature and for low concentrations (2–6 mol% of yttria), the tetragonal structure is stabilized, but this phase separates into monoclinic or cubic-doped structures as a function of temperature and time and is, therefore, called partially stabilized zirconia (PSZ). In the other hand, 8–40 mol% Y2O3 is necessary to obtain the fully stabilized cubic structure (YSZ, yttria stabilized zirconia). Over 40 mol%, the system crystallizes as a rhombohedral δ-Zr3Y4O12 phase.9–12
In addition to the structural stabilization, doping of zirconium oxide with yttria has two main consequences: it improves the thermo-mechanical properties and determines the high ionic conductivity because of the presence of oxygen vacancies in the doped material.7 The ionic conductivity, together with the number of vacancies, increases with the dopant concentration reaching a maximum at 8 mol% Y2O3 and then significantly decreases for higher dopant amount, probably because, at higher Y2O3 content, vacancies are trapped in defect complexes.13,14
The high mechanical strength, the thermal shock resistance, the low thermal conduction and the excellent oxygen ion conduction make YSZ the most common material used as electrolyte in SOFC, oxygen sensors, oxygen pumps and other electrochemical devices, mainly working at temperature higher than 600 °C.12,15–18
The mechanism of stabilization in YSZ is not completely understood and it is still subject of debate. Decreasing of the coordination number of Zr atoms from eight to a value close to seven (like in monoclinic zirconia), as a consequence of the presence of the oxygen vacancies, is the prevailing explanation, in accordance with the observation that the covalent nature of the Zr–O bond favours structures with lower coordination numbers.19 This rationalization indirectly implies that oxygen vacancies are associated to Zr4+ rather than to the Y3+ cations, contrarily to the expected electrostatic attraction between the vacancy and the dopant ions.12,16,19,20
Unfortunately, experimental studies provide conflicting data that do not give clear indication about the nature of the stabilization mechanism. Indeed some of them indicate that vacancies prefer to bind to the dopant,21,22 while others refer to a preferential association between the vacancies and Zr cations.23–27 Theoretical studies10,12,16,17,19,20,28,29 on the bulk structure of PSZ or YSZ support the latter case in which Y next-nearest neighbour positions (NNN) to the vacancy are energetically favoured with respect to the nearest neighbour (NN) ones. The configuration of Zr, O, Y and oxygen vacancies is however not decisively characterized and the proposed mechanism of stabilization still needs to be better clarified.
The structural characterization of the surfaces of this oxide is also difficult from an experimental point of view. Despite several experiments30,31 suggesting the presence of a monolayer rich in yttrium on top of a thicker undoped ZrO2 layer, there is still uncertainty about the locations of the oxygen vacancies and Y atoms.9,32
Modelling can help investigating structural and electronic properties of the oxide surfaces, providing information that is difficult to obtain experimentally. Some theoretical studies have been already devoted to the description of the structural properties of YSZ or PSZ surfaces,8,9,11,15,33,34 but there is not a systematic investigation of dopant and vacancies distribution. This would however be particularly appealing as the knowledge of the geometrical and electronic properties of YSZ surfaces is crucial for SOFC applications of this material.
For these reasons, with the aim to gain further information on the complex surface chemistry of YSZ, clarifying the structure and the impact of the doping on the electronic and chemical properties of YSZ surfaces, we performed first-principles Density Functional Theory (DFT) calculations on the most stable (111) surface of YSZ.30,35,36 Moreover, while previous theoretical works on YSZ bulk or surfaces were based on various values of dopant concentrations (from 9 to 14 or even 40 mol% of yttria), we focused on the experimental doping amount (8 mol% Y2O3) that ensures the highest conductivity and corresponds to the material used in practical SOFC applications.
In particular, we first explored the formation of isolated oxygen defects on the clean (111) ZrO2 surface. Indeed, the formation of colour centres in zirconia has been extensively described for monoclinic ZrO2 bulk20,37 and surfaces,38 for tetragonal ZrO2 bulk10,39 and surfaces,11 for cubic zirconia bulk,12,16,17 while only little information can be found on cubic surfaces.34
The arrangement of Zr, O, Y and oxygen vacancies on the (111) surface was then studied in detail through a systematic study of the stability, geometry and electronic structure of seven different configurations for Y atoms and oxygen vacancies.
II. Computational details
Calculations have been performed in the framework of DFT and using a linear combination of atomic orbitals (LCAO) approach, by means of the periodic CRYSTAL09 (ref. 40) code.The global hybrid functional PBE0,41,42 a parameter-free functional mixing 25% of HF exchange in a PBE scheme, has been selected as the exchange-correlation functional of choice. We have already successfully applied this functional to the description of structural and electronic features of zirconia bulk and surfaces.35,43
For Zr atoms, the small core ECP (effective core pseudo-potential) of Hay and Wadt44–46 was used to describe the 28 inner core electrons together with a (5sp,4d) → [3sp,2d]47 contraction scheme to describe the (4s24p65s24d2) valence electrons. The Durand–Barthelat48,49 large core ECP to replace the 1s core electrons and a (4sp) → [2sp] contraction scheme50 for the outer electrons was instead selected as basis set for O atoms. Finally, for the Y atoms, the Hay and Wadt44–46 small core ECP and the (4sp,2d) → [2sp,1d] contraction scheme50 for the (4s24p65s24d1) valence electrons were used. These basis sets have already been shown to perform closely to larger ones.35
The clean (111) surface of cubic ZrO2 was cleaved from the optimized bulk ZrO2 structure with lattice parameters a = 5.100 Å.35 In particular, it was modelled by a (2 × 2) supercell with a total of 72 atoms (Zr24O48). To study the formation of colour centres in this surface, two oxygen atoms were removed from the supercell (Zr24O46), but leaving the associated basis set centred in the atomic position of the defect site. Finally, to model the (111) surface of YSZ, in addition to the two vacancies, four Zr atoms of the supercell were replaced by four Y atoms, leading to Zr20Y4O46 and corresponding to the experimental 8 mol% Y2O3 doping amount.
For all the calculations, the reciprocal space was sampled using a Monkhorst–Pack51 grid with shrinking factors (6 × 6 × 1), corresponding to 13 points in the irreducible Brillouin zone (IBZ) of the supercell. Extrafine52 tolerances were considered for the evaluation of Coulomb and exchange series. Finally, geometry optimization were performed keeping the supercell lattice parameters (a = b = 7.196 Å, γ = 120°) constant, while all the atomic positions were allowed to relax, until the maximum and root-mean square atomic forces and displacements were simultaneously less than 4.5 × 10−4, 3.0 × 10−4, 1.8 × 10−3 and 1.2 × 10−3 au, respectively.40
The energy of formation of n vacancies (Efn) on the cubic (111) surface was computed with respect to molecular (1/2O2) or atomic (O) oxygen as, respectively:
 | (1) |
| Efn(O) = E(surface with n vacancies) + nE(O) − E(surface) | (2) |
For the (111) YSZ surface, the energy (Es−f) due to the substitution of Zr atoms with Y atoms and simultaneously to the formation of oxygen vacancies (with respect to O2 or O) was computed as:
 | (3) |
| Es−f(O) = E(doped surface) + 2nE(Zr) + nE(O) − E(surface) − 2nE(Y) | (4) |
III. Surface models
The pure cubic zirconia phase belongs to the space group Fm3m and has an fcc sublattice of zirconium atoms and oxygen atoms in the tetrahedral sites. Therefore, Zr atoms are 8-fold, while O atoms are 4-fold coordinated. YSZ bulk has also a fluorite like structure with an fcc cation sub-lattice of Zr and Y atoms and a simple cubic anion sub-lattice of O atoms and oxygen vacancies.The (111) surface was taken into account for both systems because previous calculations showed that it remains the most stable even after the introduction of dopant atoms.9,11,15,36 Moreover, following previous results, only the case of oxygen terminated surfaces was taken into account, because they are more stable than the Zr terminated ones.11
Along the normal to the (111) surface there is a sequence of charged planes: the first plane is constituted by oxygen anions, the second by zirconium cations and the third again by oxygen ions. When cubic ZrO2 crystals are cut along this direction, surface sites with three-fold coordinated oxygen and seven-fold coordinated Zr atoms are formed. In order to ensure the convergence of structural and electronic properties, the clean (111) surface of cubic ZrO2 was modelled by a (2 × 2) supercell with a depth of 18 atomic layers (six O–Zr–O trilayers) in the direction perpendicular to the surface, for a total of 72 atoms (Zr24O48, Fig. 1).
The formation of oxygen defects on the clean surface was studied removing two oxygen atoms from this supercell (Zr24O46). Bogicevic et al.12 observed that as a consequence of the repulsion between the vacancies, oxygen defects in zirconia tend to align along the [111] direction as third nearest neighbours for high (15–40 mol%) Y2O3 concentrations. They also observed that for more dilute composition the vacancies could space themselves at greater distances. The two vacancies were then placed along the [111] direction in a symmetrical way at the top and bottom of the supercell. This ensures to leave enough space between the two vacancies (∼13 Å) to avoid any spurious interactions between defects. Two possible positions for an oxygen vacancy on the (111) surface were tested. From Fig. 1, it is evident that one corresponds to a three-fold coordinated surface oxygen site (indicated as surface vacancy in the following), while the other corresponds to a four-fold coordinated bulk-like site (indicated as subsurface vacancy in the following).
Finally, to obtain a representative model for YSZ we created, as before, two oxygen vacancies, one at the top and the other at the bottom of the ZrO2 supercell and, at the same time, we substituted four Zr with four Y atoms (Zr20Y4O46). This allows mimicking the experimental percentage of 8 mol% Y2O3. One pair of Y atoms was positioned at the top and the other at the bottom of the supercell in a symmetrical way, each one in correspondence with one oxygen vacancy.
Two and five different configurations of the two Y3+ cations with respect to a surface or subsurface vacancy were taken into account, similarly to the work of Ganduglia-Pirovano et al.,53 who studied the formation of oxygen vacancies and the localization of the excess electrons on different pair of sites on the (111) surface of the cubic fluorite structure of CeO2.
There are four Zr atoms in the outermost Zr plane, three of them (1, 2, and 4 in Fig. 1) are in nearest-neighbour position (NN) to a surface vacancy, while the fourth one (3 in Fig. 1) is in a next nearest-neighbour (NNN) position with respect to it. For a subsurface vacancy, instead, Zr atoms 1, 2, and 3 of the outermost Zr layer and Zr atom 8 which belong to the plane below this defect are in NN positions, while Zr atom 4 on the plane above and Zr atoms 5, 6, and 7 in the plane below it are in NNN positions. If we consider the bulk truncated surface, NN and NNN Zr atoms are at a distance of respectively 2.22 Å and 4.20 Å away from the vacancy.
Hence for a surface vacancy, the two Y atoms can be introduced both NN to the defect or one can be NN and the other NNN to it, for a total of two possible configurations ((1,2)NN–NN and (2,3)NN–NNN). For a subsurface vacancy, the two Y atoms could be placed both NN to the defect, or one in NN and the other in NNN positions or they can be both in NNN sites, for a total of five different configurations ((1,2)NN–NN), (2,8)NN–NN), (1,4)NN–NNN, (4,8)NNN–NN, and (4,6)NNN–NNN).
IV. Results and discussion
A. Isolated oxygen vacancy on (111) ZrO2 surface
As a preliminary step toward the study of YSZ, the stability, the structural and electronic properties of the zirconia (111) surface containing only isolated oxygen vacancies were investigated.When a neutral oxygen atom is removed, two electrons associated to an oxygen anion are left behind in the lattice and a neutral oxygen vacancy (VO) is formed, with two possible spin states for the resulting system.
The energy of formation (Ef) of one surface or subsurface oxygen vacancy on the (111) surface was computed and reported in Table 1. Ef was calculated with respect to atomic oxygen (O) or molecular oxygen (O2). The second species is generally preferred as reference in more recent works, because molecular oxygen is used in some oxidation techniques providing the right reference for the chemical potential and because the degeneracy of the ground state of triplet for the O atom is poorly described in DFT.37
| Vacancy | Spin State | Ef (1/2 O2) | Ef (O) | |
|---|---|---|---|---|
| a See ref. 39. Computed for bulk t-ZrO2.b See ref. 38. Computed for bulk m-ZrO2.c See ref. 37. Computed for bulk m-ZrO2.d See ref. 34. Computed for c(111). | ||||
| This work | Surface | S | 8.4 | 10.2 |
| T | 8.5 | 10.3 | ||
| Subsurface | S | 7.0 | 8.7 | |
| T | 7.3 | 9.1 | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Other works | ||||
| GTO/B3LYP | 7.2a | — | ||
| PAW/PBE | 5.9b | 8.9b | ||
| PAW/GGA-II | — | 8.9c | ||
| PAW/PBE | — | 8.8d | ||
The obtained values are in agreement with the ones reported in previous theoretical works, which, however, refer to the bulk structure of the tetragonal and monoclinic zirconia phases or to the cubic (111) surface,34 even if in this latter case the role of different oxygen sites was not taken into account.
The singlet state is always found to be the most stable independently of the type of vacancy: it is more stable than the triplet one by 0.3 eV and 0.1 eV for a 4-fold coordinated subsurface and for the 3-fold coordinated surface vacancy, respectively. Considering the difference in stability, the following discussion is based only on the most stable singlet state.
Subsurface vacancies resulted to be the most stable, with formation energies of 1.4 and 1.5 eV (with respect to O2 and O, respectively) lower than the corresponding energies for the surface one. Indeed, Syzgantseva et al.38 already reported that even if surfaces are more reducible than bulk, due to the decrease in coordination of surface atoms, the most stable vacancy does not always correspond to those created from under-coordinated oxygen site.
The formation energy of the oxygen vacancy can be considered as a measure of the reducibility of the material. These values show that the reduction behaviour of ZrO2 surfaces from the point of view of energetics is closer to the one of irreducible oxides like MgO (Ef(O) ∼ 10.6 eV and 9.8 eV for surfaces38) rather than to the one of a reducible oxide such as TiO2 (Ef(O) ∼ 7 eV for the bulk and 5.5–6 eV for surfaces).38 The irreducible nature of ZrO2 is thus confirmed.
Optimized geometries for the system with a surface or subsurface vacancy are reported in Fig. 2. Structural rearrangements are generally restricted to atoms in NN positions to the vacancy for both a surface and subsurface defect, in particular to NN oxygen atoms. Again, this is similar to a metal oxide like MgO, where F centres are stable and their formation is accompanied by small relaxation of the O atom NN to the defect, indicating electron trapping in the vacancies.16,38 The surface vacancy is associated to the largest displacements, which cause the NN O atoms to move toward the vacancy and NN Zr atoms to move radially away from it to a distance of 2.39 Å (the distance of NN Zr atoms from the vacancy for the unrelaxed system is 2.22 Å). Smaller relaxations are observed for the most stable system with subsurface vacancies (see Table 2).
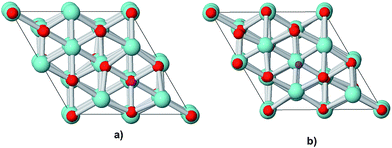 | ||
| Fig. 2 Top view of the supercell for the optimized geometry of the (111) surface with (a) surface or (b) subsurface vacancies. | ||
| Vacancy | Label | x | y | z |
|---|---|---|---|---|
| Surface | Oc(NN) | 0.21 | 0.12 | 0.00 |
| Od(NN) | 0.00 | −0.24 | 0.00 | |
| Ob(NN) | −0.21 | 0.12 | 0.00 | |
| Oe(NNN) | 0.00 | −0.10 | −0.02 | |
| Zr1(NN) | 0.03 | 0.02 | −0.05 | |
| Zr2(NN) | 0.00 | −0.04 | −0.05 | |
| Zr3(NNN) | 0.00 | 0.00 | 0.11 | |
| Zr4(NN) | −0.03 | 0.02 | −0.05 | |
| Subsurface | Oe(NN) | −0.09 | −0.10 | −0.07 |
| Of(NN) | 0.04 | −0.13 | −0.08 | |
| Oa(NN) | 0.09 | −0.06 | 0.02 | |
| Oc(NNN) | 0.05 | 0.07 | −0.03 | |
| Zr1(NN) | 0.00 | −0.09 | −0.02 | |
| Zr2(NN) | 0.06 | 0.03 | −0.02 | |
| Zr3(NN) | 0.07 | −0.04 | 0.04 | |
| Zr4(NNN) | 0.00 | 0.00 | −0.08 |
There could be a relationship between relaxation and charge redistribution: the lower stability of the surface vacancies is accompanied by greater relaxation, which is in turn accompanied by a greater redistribution of charge density. Table 3 shows that, on the NN Zr atoms, the atomic charge decreases from +2.17 for the clean surface to +1.97 and +2.01 for a surface and a subsurface vacancy, respectively. Smaller decreases are observed for Zr atoms NNN to the vacancy.
| Label | Clean | Oxygen vacancy | |
|---|---|---|---|
| Surface | Subsurface | ||
| Oe | −1.06 | −1.06 | −1.06 |
| Of | −1.06 | −1.06 | −1.06 |
| Oa | −1.06 | −0.35 | −1.06 |
| Oc | −1.12 | −1.11 | −1.12 |
| Od | −1.12 | −1.11 | −1.12 |
| Ob | −1.12 | −1.11 | −0.46 |
| Zr1 | 2.17 | 1.97 | 2.01 |
| Zr2 | 2.17 | 1.97 | 2.01 |
| Zr3 | 2.17 | 2.06 | 2.01 |
| Zr4 | 2.17 | 1.97 | 2.17 |
| Zr8 | 2.21 | 2.21 | 2.04 |
The band structure and the total and atom-projected density of states (DOS) of the cubic (111) surface in Fig. 3 show a wide band between 0 and about −7 eV, which constitutes the top of the valence band (VB) and corresponds to the O-2p states. An appreciable contribution of the Zr-4d states to this band can also be seen from the density of states, indicating the presence of covalent bonding in the solid. The bottom of the conduction band (CB), between 5 and 12 eV, is instead made by the Zr-4d states that are clearly split into two groups, the low energy eg and the high energy t2g states, as a consequence of the crystal field associated to the cubic environment (Oh point group symmetry) of the surrounding oxygen ions.
 | ||
| Fig. 3 Band structure (a) and total and atom-projected density of states (b) for the clean (111) ZrO2 surface. | ||
The computed band gap is 5.48 eV, in line with previous theoretical calculations for ZrO2 bulk and surfaces17,35,54,55 and within the range of experimental values, which spread between 4 (EELS56) and 6 eV (VUV57). This large range of results (2 eV) could be due from one side to an overestimation of the VUV methods57 and from the other to the attribution of the low energy part of the EELS states to transitions from the VB to still unassigned localized states in the gap, due to extrinsic defects (defect states or oxygen vacancy) or induced by the disorder in the YSZ solid solution.57,58
Reduction via surface or subsurface vacancy formation leads to the creation of a defect state in the gap, (see Fig. 4). The two left electrons, which filled the O-sp states, now occupy a newly created vacancy state. Therefore, a new doubly occupied defect energy level appears in the band gap. As we can see from the DOS, it is mostly made by Zr-4d states, so we can imagine that after the removal, these two electrons are moving back from O to Zr, occupying a linear combination of the d orbitals of neighbouring Zr atoms.17 A small contribution of O-2p states could also be observed in Fig. 4. This defect state is localized in the vacancy region. It resembles a neutral VO center in alkaline-earth oxides and has the typical structure of an F-center.16,38,39
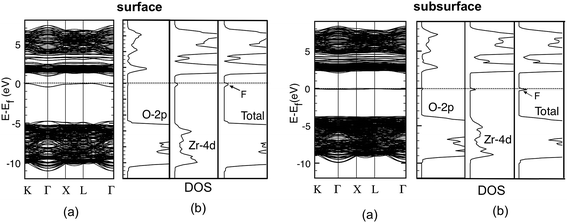 | ||
| Fig. 4 Band structure (a) and total and atom-projected density of states (b) for the system with surface (left) or subsurface (right) oxygen vacancies. | ||
The main difference in the electronic structure of a surface or subsurface vacancy, except for the slightly more flat band structure of the latter system, is in the position of the defect state, which depends on the coordination of the site from which the oxygen atom is removed. For a surface vacancy, the defect level is 4.30 eV above the top of the VB and only 0.92 eV under the bottom of the CB. For the most stable subsurface vacancy, instead, the defect level is 3.58 eV above the top of VB and 2.07 eV under the bottom of the CB (see Table 4). Therefore, the presence of the vacancy state in the gap decreases the effective band gap, which results to be higher in the case of the most stable type of defect, compared to the other surface case.
| Vacancy | Relative to VB | Relative to CB | Eg |
|---|---|---|---|
| Surface | 4.30 | 0.92 | 5.66 |
| Subsurface | 3.58 | 2.07 | 5.79 |
Finally, from Fig. 5, it is interesting to note that the defect state is clearly observed both in the DOS of the unrelaxed system, i.e. the structure in which the atoms are in the centro-symmetric fluorite positions, and in that of the relaxed one, i.e. the structure obtained after the geometry optimization.
B. Y2O3-doped (111) surface
When ZrO2 is doped with Y2O3, for each pair of aliovalent Y3+ ions that substitutes a pair of Zr4+ cations, one oxygen vacancy must be created. Hence, since Y has one d-electron less than Zr, this corresponds removing one oxygen atom together with two electrons (O2−). For this reason, the corresponding vacancy should be a doubly positively charged vacancy (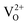 ) also called F2+ centre.
) also called F2+ centre.
In Table 5, the energy due to the creation of the oxygen vacancies and to the simultaneous substitution of Zr with Y atoms (Es−f) was reported for all the considered configurations of the four Y3+ cations with respect to a surface or subsurface vacancy (Fig. 6). According to eqn (3) and (4), the negative sign of the computed Es−f values indicate that the doping with Y2O3 stabilizes the surface.
| Vacancy | Configuration | Ef (1/2O2) | Ef (O) |
|---|---|---|---|
| Surface | (1,2)NN–NN | −28.52 | −24.98 |
| (2,3)NN–NNN | −31.14 | −27.59 | |
| subsurface | (1,2)NN–NN | −29.43 | −25.89 |
| (2,8)NN–NN | −29.98 | −26.43 | |
| (1,4)NN–NNN | −31.08 | −27.53 | |
| (4,8)NNN–NN | −30.94 | −27.40 | |
| (4,6)NNN–NNN | −32.05 | −28.50 |
In particular the preferred configuration for the surface vacancy is (2,3)NN–NNN. Indeed, for this kind of vacancy there is only one possible NNN position for the Y ions in the outermost zirconium plane and the (2,3)NN–NNN configuration results to be the most stabilized one.
For a subsurface vacancy, we found the following stabilization order: (1,2)NN–NN < (2,8)NN–NN < (4,8)NNN–NN < (1,4)NN–NNN < (4,6)NNN–NNN. Again, the preferred configuration is when the Y3+ ions are both NNN to the vacancy and, thus, the vacancy is likely to be bound to Zr4+ ions rather than to the dopant. Moreover, the most stable subsurface configuration ((4,6)NNN–NNN), with two Y in NNN position, is 0.91 eV more stable than the most stable surface one.
In summary, subsurface vacancies are favoured and Y3+ ions occupy preferentially positions NNN to the defect, in agreement with experimental observations23–27 and with previous YSZ bulk calculations.12,17
The observed stability order can be rationalized considering two different aspects related to the doping of zirconia with aliovalent Y2O3. On one hand, relative to the host lattice ions Zr4+ and O2−, the dopant ion Y3+ and the vacancy  can be seen as point defect charge −1 and +2, respectively, so that electrostatic interactions should be predominant. Coulomb considerations thus suggest that Y would most likely be NN to the vacancy. On the other hand, it was observed that the covalent nature of Zr–O bonds favours structure with lower Zr coordination, explaining in this way the higher stability at room temperature of the monoclinic ZrO2 phase (where Zr atoms are 7-fold coordinated) over the tetragonal and cubic zirconia phases (where Zr atoms are 8-fold coordinated). Thus, if in presence of the vacancies, Zr atoms prefer to be NN to the defects while the Y ions prefer NNN positions, in YSZ there will be a reduction of the average zirconium coordination from 8 to a value closer to 7, similar to the value of monoclinic zirconia. This lowering of the Zr coordination is then at the base of the mechanism of stabilization of cubic zirconia through Y2O3-doping. Moreover, in a very simple view, Y3+ has a larger radius than Zr4+ and could thus prefer the eight-fold coordination, whereas the smaller Zr4+ can better accommodate the contraction of the lattice in the neighbourhood of the vacancy that accompanies its formation. We can then conclude that the stabilization and the preference for defect sites in YSZ is a consequence of a delicate compromise between size effects and electrostatic interactions.
can be seen as point defect charge −1 and +2, respectively, so that electrostatic interactions should be predominant. Coulomb considerations thus suggest that Y would most likely be NN to the vacancy. On the other hand, it was observed that the covalent nature of Zr–O bonds favours structure with lower Zr coordination, explaining in this way the higher stability at room temperature of the monoclinic ZrO2 phase (where Zr atoms are 7-fold coordinated) over the tetragonal and cubic zirconia phases (where Zr atoms are 8-fold coordinated). Thus, if in presence of the vacancies, Zr atoms prefer to be NN to the defects while the Y ions prefer NNN positions, in YSZ there will be a reduction of the average zirconium coordination from 8 to a value closer to 7, similar to the value of monoclinic zirconia. This lowering of the Zr coordination is then at the base of the mechanism of stabilization of cubic zirconia through Y2O3-doping. Moreover, in a very simple view, Y3+ has a larger radius than Zr4+ and could thus prefer the eight-fold coordination, whereas the smaller Zr4+ can better accommodate the contraction of the lattice in the neighbourhood of the vacancy that accompanies its formation. We can then conclude that the stabilization and the preference for defect sites in YSZ is a consequence of a delicate compromise between size effects and electrostatic interactions.
Larger relaxations (between 0.3 and 1 Å) of the oxygen atoms NN to the vacancy with respect to the ones observed for the formation of oxygen vacancies on the pure (111) surface are observed. After the NN O atoms, the Zr atoms NN to the vacancies have the greatest displacements, while the Y atoms are associated to the smallest relaxations. Consequently, Zr atoms NN to the vacancy move away from it, while NN O atoms move toward the defect determining compression regions in the lattice near the defects. Generally, less stable configurations relax more. For Y3+ ions that are NN to defect sites, the average Y–O bond length is about 2.0 Å, while for dopant ions NNN to the vacancy it is of about 2.3 Å, very close to the value of the Y–O bond in Y2O3.59 Considering that Y3+ ions are larger than Zr4+ ions, this confirms that Y3+ in NN position are compressed.
| Vacancy | Configuration | Eg |
|---|---|---|
| Surface | (1,2)NN–NN | 5.71 |
| (2,3)NN–NNN | 5.65 | |
| Subsurface | (1,2)NN–NN | 5.49 |
| (2,8)NN–NN | 5.67 | |
| (1,4)NN–NNN | 5.61 | |
| (4,8)NNN–NN | 5.74 | |
| (4,6)NNN–NNN | 5.80 |
Band structure and total and atom-projected DOS of the different configurations (Fig. 7) are alike and show similar properties to the ones of the pure system: the VB is constituted by O-2p states, while the bottom of the CB is associated mainly to Zr-4d. However, there are two main differences. The first one is that Y-4d states also contribute to the bottom of the CB, but they lie at higher energies with respect to the corresponding Zr states. Moreover, it can be observed that when the Y atoms are in NNN position with respect to the vacancy, these Y-4d states are at even higher energies. This means that a stronger interaction with the vacancy determines a higher stabilization of Y-4d orbitals. The second difference is that the eg − t2g splitting of the Zr-4d states is present, especially in the least stable configuration (1,2)NN–NN, but a broadening can be observed as a consequence of the doping and the disorder that the doping creates in the structure.
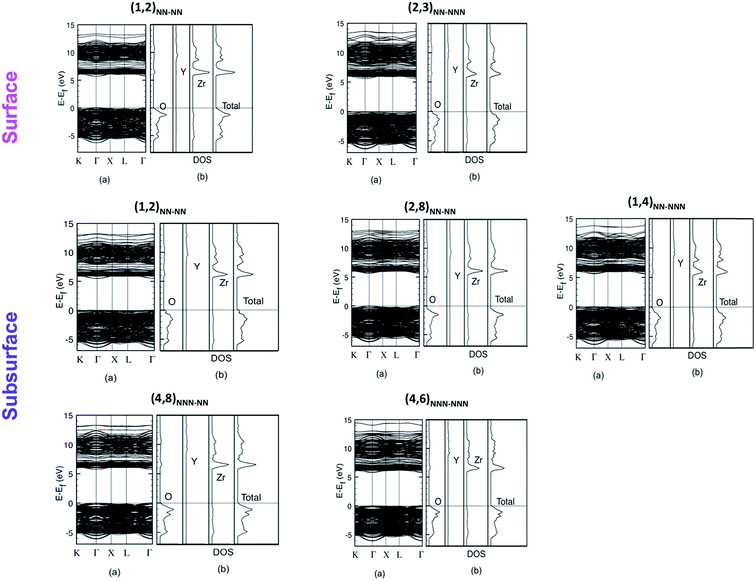 | ||
| Fig. 7 Band structure (a), total and atom-projected DOS (b) of the different configurations of the YSZ (111) surface. | ||
Although an isolated peak, corresponding to an empty colour center ( ) is present in the gap of the unrelaxed YSZ structure for each configuration, no such empty electronic state is observed in the gap of any optimized configuration (see for example the DOS for the unrelaxed and relaxed (4,6)NNN–NNN configuration in Fig. 8). This result can be explained considering the perturbation in the periodic electrostatic Madelung potential due to the presence of
) is present in the gap of the unrelaxed YSZ structure for each configuration, no such empty electronic state is observed in the gap of any optimized configuration (see for example the DOS for the unrelaxed and relaxed (4,6)NNN–NNN configuration in Fig. 8). This result can be explained considering the perturbation in the periodic electrostatic Madelung potential due to the presence of  , which increases the potential on the NN sites lowering the energy of the d states of the ions in that position with respect to the energy of the d states of cations far from the defect. This is why a mid-gap empty state is observed in the band structure and DOS of the unrelaxed system. The large relaxations of oxygen atoms NN to the vacancies generate a Madelung field that prevents the formation of the empty electronic state in the gap, compensating the perturbation of the potential in the neighbourhood of the vacancy and thus pushing the d states back in the CB (Fig. 9).16,37 The deviation from the ideal cubic symmetry is then crucial for the understanding of the electronic properties of YSZ.
, which increases the potential on the NN sites lowering the energy of the d states of the ions in that position with respect to the energy of the d states of cations far from the defect. This is why a mid-gap empty state is observed in the band structure and DOS of the unrelaxed system. The large relaxations of oxygen atoms NN to the vacancies generate a Madelung field that prevents the formation of the empty electronic state in the gap, compensating the perturbation of the potential in the neighbourhood of the vacancy and thus pushing the d states back in the CB (Fig. 9).16,37 The deviation from the ideal cubic symmetry is then crucial for the understanding of the electronic properties of YSZ.
 | ||
| Fig. 8 Total and atom-projected DOS for the relaxed (plain line) and unrelaxed (dotted line) (4,6)NNN–NNN configuration of YSZ. | ||
V. Conclusions
In this work, the formation of oxygen defects on the (111) surface of cubic zirconia as well as the stabilization of this surface through doping with the aliovalent Y2O3 oxide were investigated in detail by periodic DFT calculations. Two possible vacancy positions and two possible spin states for each position were taken into account to study the isolated oxygen vacancies on the clean (111) surface, as well as seven different configurations of Y atoms and oxygen vacancies for the Y2O3-doped (111) surface.Concerning neutral oxygen vacancy (VO) formed after the removal of O atoms from the clean surface, formation energies ranging between 8 and 10 eV were obtained. These values are close to the ones of irreducible oxide like MgO. The type of site from which the O atom is removed seems also to play an important role, as subsurface vacancies are found to be ∼1.4–1.5 eV more stable than surface ones. Small relaxation accompanies the formation of VO, suggesting that the two electrons left behind by the removed oxygen atoms are trapped in the vacancy vicinity. Indeed, surface vacancies, which are less stable and relax more in comparison to subsurface one, are also associated to a greater redistribution of charge density. As a consequence of the electrons occupying the vacancy state, a doubly occupied defect energy level appears in the band gap of ZrO2. These results evidence the different intrinsic conduction properties of zirconia, an ionic conductor, with respect to other oxides, that can be used in composites electrolytes for LT-SOFC applications, as CeO2. This irreducible nature is important since CeO2 can suffer, in SOFC operating conditions, of partial reduction of Ce4+ ions into Ce3+ cations, becoming a mixed electron/ion conductor. Naturally, these differences should be taken into account when comparing the properties and performances of different composite in order to elucidate the conduction properties of different composite materials.
As regards the doping with yttria, the computed values for the energy due to the creation of the oxygen vacancies ( ) and simultaneously to the substitution of Zr with Y atoms (Es−f) confirm, as expected, that doping of the (111) zirconia surface with Y2O3 is accompanied by the stabilization of the system. Subsurface vacancies are favoured also in this case and Y ions occupy preferentially the positions NNN to the defect. Indeed, of all the tested configurations, the (4,6)NNN–NNN, with a subsurface vacancy and two Y atoms both in NNN position to the defect, is found to be by far the most stable. Consequently, Zr4+ ions should prefer to be NN to the vacancies, with the consequent reduction of their coordination number, which supports the idea that the stabilization mechanism is related to the lowering of the coordination of Zr atoms. Moreover, upon vacancy formation, the lattice contraction is easier when the NN ions are Zr4+ cations rather then the larger Y3+ cations, which instead prefer higher coordination numbers. Large relaxations are observed for all the studied configurations. They are associated to the O atoms NN to the vacancies, which move toward the vacancy, thus determining the contraction of the lattice in the defect region. This deviation from the ideal cubic geometry is fundamental to explain the electronic properties of the system and can be rationalized by electrostatic potential arguments.
) and simultaneously to the substitution of Zr with Y atoms (Es−f) confirm, as expected, that doping of the (111) zirconia surface with Y2O3 is accompanied by the stabilization of the system. Subsurface vacancies are favoured also in this case and Y ions occupy preferentially the positions NNN to the defect. Indeed, of all the tested configurations, the (4,6)NNN–NNN, with a subsurface vacancy and two Y atoms both in NNN position to the defect, is found to be by far the most stable. Consequently, Zr4+ ions should prefer to be NN to the vacancies, with the consequent reduction of their coordination number, which supports the idea that the stabilization mechanism is related to the lowering of the coordination of Zr atoms. Moreover, upon vacancy formation, the lattice contraction is easier when the NN ions are Zr4+ cations rather then the larger Y3+ cations, which instead prefer higher coordination numbers. Large relaxations are observed for all the studied configurations. They are associated to the O atoms NN to the vacancies, which move toward the vacancy, thus determining the contraction of the lattice in the defect region. This deviation from the ideal cubic geometry is fundamental to explain the electronic properties of the system and can be rationalized by electrostatic potential arguments.
In conclusion, this systematic investigation of the dopant and vacancies arrangement on the (111) surface allowed us to clarify the effect of the doping on the structural properties of the YSZ (111) surface, to elucidate the proposed mechanism of stabilization, and to explain the electronic features of YSZ where oxygen vacancies, that determine the ionic conductivity of the material, are present but mid-gap states are not observed. Finally, this work allowed to find a model of the YSZ (111) surface (i.e. the most stable arrangement with Y atoms and oxygen in (4,6)NNN–NNN configuration) that is reliable (it is derived from a detailed comparison of the structural and electronic features of different configurations) and that has the advantage of reproducing the experimental 8 mol% Y2O3 amount. This model could be used in future studies, for example to simulate the oxide-carbonate interface of composite electrolytes, in order to further elucidate the basic operating principles of LT-SOFCs.
References
- R. M. Ormerod, Chem. Soc. Rev., 2003, 32, 17–28 RSC.
- X. Wang, Y. Ma and B. Zhu, Int. J. Hydrogen Energy, 2012, 37, 19417–19425 CrossRef CAS PubMed.
- X. Wang, Y. Ma, S. Li, A.-H. Kashyout, B. Zhu and M. Muhammed, J. Power Sources, 2011, 196, 2754–2758 CrossRef CAS PubMed.
- M. Benamira, A. Ringuedé, L. Hildebrandt, C. Lagergren, R.-N. Vannier and M. Cassir, Int. J. Hydrogen Energy, 2012, 37, 19371–19379 CrossRef CAS PubMed.
- M. Benamira, A. Ringuedé, V. Albin, R.-N. Vannier, L. Hildebrandt, C. Lagergren and M. Cassir, J. Power Sources, 2011, 196, 5546–5554 CrossRef CAS PubMed.
- A. S. V. Ferreira, T. Saradha, F. L. Figueiredo and F. M. B. Marques, Int. J. Energy Res., 2011, 35, 1090–1099 CrossRef CAS.
- N. Clausen, M. Ruhle and A. H. Heuer, in Advances in Ceramics, The American Ceramic Society, Columbous, OH, 1984, vol. 12 Search PubMed.
- X. Xia, R. J. Oldman and C. R. A. Catlow, J. Mater. Chem., 2011, 21, 14549–14558 RSC.
- X. Xia, R. Oldman and R. Catlow, Chem. Mater., 2009, 21, 3576–3585 CrossRef CAS.
- A. Eichler, Phys. Rev. B: Condens. Matter Mater. Phys., 2001, 64, 174103–174108 CrossRef.
- A. Eichler and G. Kresse, Phys. Rev. B: Condens. Matter Mater. Phys., 2004, 69, 045402–045408 CrossRef.
- A. Bogicevic, C. Wolverton, G. Crosbie and E. Stechel, Phys. Rev. B: Condens. Matter Mater. Phys., 2001, 64, 014106–014114 CrossRef.
- R. Devanathan, W. Weber, S. Singhal and J. Gale, Solid State Ionics, 2006, 177, 1251–1258 CrossRef CAS PubMed.
- R. Krishnamurthy, Y.-G. Yoon, D. J. Srolovitz and R. Car, J. Am. Ceram. Soc., 2005, 87, 1821–1830 CrossRef PubMed.
- G. Ballabio, M. Bernasconi, F. Pietrucci and S. Serra, Phys. Rev. B: Condens. Matter Mater. Phys., 2004, 70, 075417–75426 CrossRef.
- G. Stapper, M. Bernasconi, N. Nicoloso and M. Parrinello, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 59, 797–810 CrossRef CAS.
- B. Králik, E. Chang and S. Louie, Phys. Rev. B: Condens. Matter Mater. Phys., 1998, 57, 7027–7036 CrossRef.
- A. D. Mayernick, M. Batzill, A. C. T. van Duin and M. J. Janik, Surf. Sci., 2010, 604, 1438–1444 CrossRef CAS PubMed.
- V. Stefanovich, A. L. Shluger and C. R. Catlow, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 49, 11560–11571 CrossRef.
- S. Fabris, A. T. Paxton and M. W. Finnis, Acta Mater., 2002, 50, 5171–5178 CrossRef CAS.
- W. L. Roth, R. Wong, A. I. Goldman, E. Canova, Y. H. Kao and B. Dunn, Solid State Ionics, 1986, 18 & 19, 1115–1119 Search PubMed.
- D. Steele and B. E. F. Fender, J. Phys. Chem. Solids, 1974, 7, 1–11 CAS.
- C. R. A. Catlow, A. V. Chadwick, G. N. Greaves and L. M. Moroney, J. Am. Ceram. Soc., 1986, 69, 272–277 CrossRef CAS PubMed.
- B. W. Veal, A. G. McKale, A. P. Paulikas, S. J. Rothman and L. J. Nowicki, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 150, 234–240 CAS.
- P. Li, I. Chen and J. E. Penner-Hahn, Phys. Rev. B: Condens. Matter Mater. Phys., 1993, 48, 10082–10089 CrossRef CAS.
- P. Li, I. Chen and J. E. Penner-Hahn, Phys. Rev. B: Condens. Matter Mater. Phys., 1993, 48, 10074–10081 CrossRef CAS.
- P. Li, I. Chen and J. E. Penner-Hahn, Phys. Rev. B: Condens. Matter Mater. Phys., 1993, 48, 10063–10073 CrossRef CAS.
- L. Jin, Q. Yu, A. Rauf and C. Zhou, Solid State Sci., 2012, 14, 106–110 CrossRef CAS PubMed.
- G. P. Cousland, R. A. Mole, M. M. Elcombe, X. Y. Cui, A. E. Smith, C. M. Stampfl and A. P. J. Stampfl, J. Phys. Chem. Solids, 2014, 75, 351–357 CrossRef CAS PubMed.
- C. Morterra, G. Cerrato and L. Ferrato, Mater. Chem. Phys., 1994, 37, 243–251 CrossRef CAS.
- M. De Ridder, R. G. Van Welzenis, H. H. Brongersma and U. Kreissig, Solid State Ionics, 2003, 158, 67–77 CrossRef CAS.
- A. Bernasik, K. Kowalski and A. Sadowski, J. Phys. Chem. Solids, 2002, 63, 233–239 CrossRef CAS.
- X. Xia, R. J. Oldman and C. R. A. Catlow, J. Mater. Chem., 2012, 22, 8594–8612 RSC.
- M. Shishkin and T. Ziegler, J. Phys. Chem. C, 2008, 112, 19662–19669 CAS.
- C. Ricca, A. Ringuedé, M. Cassir, C. Adamo and F. Labat, J. Comput. Chem., 2015, 36, 9–21 CrossRef CAS PubMed.
- A. Christensen and E. Carter, Phys. Rev. B: Condens. Matter Mater. Phys., 1998, 58, 8050–8064 CrossRef CAS.
- A. Foster, V. Sulimov, F. Lopez Gejo, a. Shluger and R. Nieminen, Phys. Rev. B: Condens. Matter Mater. Phys., 2001, 64, 224108 CrossRef.
- O. A. Syzgantseva, M. Calatayud and C. Minot, J. Phys. Chem. C, 2012, 116, 6636–6644 CAS.
- C. Gionco, M. C. Paganini, E. Giamello, R. Burgess, C. Di Valentin and G. Pacchioni, Chem. Mater., 2013, 25, 2243–2253 CrossRef CAS.
- R. Dovesi, V. R. Saunders, C. Roetti, R. Orlando, C. M. Zicovich-Wilson, F. Pascale, B. Civalleri, K. Doll, N. M. Harrison, I. J. Bush, P. D'Arco and M. Llunell, CRYSTAL09 User's Manual, University of Torino, Torino, 2009 Search PubMed.
- C. Adamo and V. Barone, J. Chem. Phys., 1999, 110, 6158–6170 CrossRef CAS PubMed.
- M. Ernzerhof and G. E. Scuseria, J. Chem. Phys., 1999, 110, 5029–5036 CrossRef CAS PubMed.
- Y. L. Yang, X. L. Fan, C. Liu and R. X. Ran, Phys. Rev. B: Condens. Matter Mater. Phys., 2014, 434, 7–13 CrossRef CAS PubMed.
- P. J. Hay and W. R. Wadt, J. Chem. Phys., 1985, 82, 299–310 CrossRef CAS PubMed.
- W. R. Wadt and P. J. Hay, J. Chem. Phys., 1985, 82, 284–298 CrossRef CAS PubMed.
- P. J. Hay and W. R. Wadt, J. Chem. Phys., 1985, 82, 270–283 CrossRef CAS PubMed.
- T. Bredow and M. Lerch, Zeitschrift für Anorg. und Allg. Chemie, 2004, 630, 2262–2266 CrossRef.
- J. C. Barthelat, P. Durand and A. Serafini, Mol. Phys., 1977, 33, 159 CrossRef CAS.
- J. C. Barthelat and P. Durand, Gazz. Chim. Ital., 1978, 108, 225–236 Search PubMed.
- Basis set available at the following web site, http://www.crystal.unito.it/basis-sets.php.
- H. J. Monkhorst and J. D. Pack, Phys. Rev. B: Condens. Matter Mater. Phys., 1976, 13, 5188–5192 CrossRef.
- The default grid in CRYSTAL09 has 55 radial points and a maxiumum 434 angular points, while the extrafine integration scheme corresponds to 75 radial points and 975 angular points. See ref. 40.
- M. Ganduglia-Pirovano, J. Da Silva and J. Sauer, Phys. Rev. Lett., 2009, 102, 026101–026104 CrossRef.
- F. Gallino, C. Di Valentin and G. Pacchioni, Phys. Chem. Chem. Phys., 2011, 13, 17667–17675 RSC.
- T. Bredow, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 75, 144102–144111 CrossRef.
- D. McComb, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 7094–7102 CrossRef CAS.
- R. French, S. Glass, F. Ohuchi, Y. Xu and W. Ching, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 49, 5133–5142 CrossRef CAS.
- L. Dash, N. Vast, P. Baranek, M.-C. Cheynet and L. Reining, Phys. Rev. B: Condens. Matter Mater. Phys., 2004, 70, 245116–245117 CrossRef.
- N. Thromat, C. Noguerra, M. Gautier, F. Jollet and J. P. Duraud, Phys. Rev. B: Condens. Matter Mater. Phys., 1991, 44, 7904–79011 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |




