Synthesis of carbon xerogel nanoparticles by inverse emulsion polymerization of resorcinol–formaldehyde and their use as anode materials for lithium-ion battery
Abstract
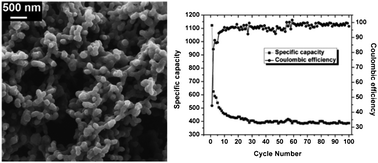
Carbon xerogel nanoparticles were synthesized using repeated inverse emulsion polymerization of resorcinol–formaldehyde, followed by subcritical drying and pyrolysis at 1173 K. The prepared carbon xerogel nanoparticles were then structurally characterized by scanning electron microscopy, Raman spectroscopy, X-ray diffraction, transmission electron microscopy and small angle X-ray scattering. Further, these carbon xerogel nanoparticles were tested for their electrochemical properties. Galvanostat charge/discharge experiments revealed a reversible capacity (400 mA h g−1) higher than that of graphite with excellent capacity retention and coulombic efficiency. Cyclic voltammetry and impedance spectroscopic studies were also carried out to support these findings. The remarkable electrochemical behaviour as exhibited by these carbon xerogel nanoparticles paves the way for their potential use as anode materials in lithium-ion battery.

 Please wait while we load your content...
Please wait while we load your content...