MgAl layered double hydroxides with chloride and carbonate ions as interlayer anions for removal of arsenic and fluoride ions in water†
Pei-Pei Huangab,
Chang-Yan Cao*a,
Fang Weia,
Yong-Bin Suna and
Wei-Guo Song*a
aBeijing National Laboratory for Molecular Sciences (BNLMS), Key Laboratory of Molecular Nanostructure and Nanotechnology, Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190, People's Republic of China. E-mail: cycao@iccas.ac.cn; wsong@iccas.ac.cn
bUniversity of Chinese Academy of Sciences, Beijing 100049, People's Republic of China
First published on 6th January 2015
Abstract
Layered double hydroxides (LDHs) are regarded as effective adsorbents to remove arsenic and fluoride ions in water. Compared to thorough research on calcined LDHs, very few works have focused on the adsorption properties and mechanisms of LDHs themselves for these ions. In this manuscript, three-dimensional hierarchical flower-like MgAl layered double hydroxides (MgAl-LDHs) with chloride and carbonate ions as interlayer anions were synthesized via a solvothermal method without any surfactant. When tested as adsorbents for removal of As(V) and F− ions, these hierarchical MgAl-LDHs showed maximum capacities of 125.8 and 28.6 mg g−1, respectively, under neutral conditions. The adsorption mechanisms for As(V) and F− of hierarchical MgAl-LDHs were elucidated by X-ray diffraction patterns, Fourier transformed infrared spectra, X-ray photoelectron spectroscopy and energy dispersive spectra. The results suggested that ion exchange between interlayer anions in hierarchical MgAl-LDHs and As(V) or F− ions was the main adsorption mechanism. Chloride and carbonate ions intercalated in the MgAl-LDHs layers were exchanged with As(V), while only chloride ions were exchanged with F− during adsorption.
Introduction
Arsenics and fluoride are serious health risk for many residents in contaminated areas. Among various water treatment methods, adsorption may be one of the most adopted ones.1–14 Recently, layered double hydroxides (LDHs) and calcined LDHs (CLDHs) have been demonstrated as effective adsorbents to remove organic dyes and heavy metal anions due to advantages of low cost, thermal stabilities and excellent adsorption capacities.15–21 The general formula of LDHs can be expressed as [MII1−xMIIIx(OH)2]x+[An−x/n]·mH2O, where MII and MIII are di- and trivalent metal cations in the octahedral positions of brucite-like layers, yielding excessive positive charge and An− represents an n-valent anion which balances the positive charges on the layers.22 Among various morphologies, three-dimensional nanostructures that are composed of nanometer-sized building blocks show many advantages in adsorption.23–28 Their nanometer-sized building blocks provide high surface area and active sites for adsorption and the overall micrometer-sized structure provides desirable mechanical strength and easy recovery. For example, Chen et al. reported a kind of SiO2@LDH hierarchical spheres via ultrasound assisted direct growth of MgAl-LDH nanosheets on the surface of silica spheres for efficient removal of pharmaceutical waste from water.29 Zhou prepared Li/Al-LDH with hierarchical porous structures by a hydrothermal method to remove fluoride ions.19 However, only very few works reported about three-dimensional hierarchically LDHs and calcined LDHs.Many previous works paid attention mainly on the removal of arsenate and fluoride ions over CLDHs, and the adsorption mechanism was mainly proposed to the structure reconstruction of CLDHs.18,19,30–32 Yu et al. prepared 3D hierarchical flower-like MgAl-LDHs via a solvothermal method and they proposed that the main adsorption mechanism for As(V)/Cr(VI) removal was the structure reconstruction mechanism of MgAl-CLDHs.31 Zhou reported that hierarchically porous Li/Al-CLDHs exhibited strong affinity towards fluoride via ion exchange, physical adsorption as well as insertion into the host layer lattice during the rehydration process.19 However, very few works focused on the LDHs themselves, including adsorption properties and mechanisms.
Ion exchange (such as hydroxyl group, carbonate, and so on) is a dominant adsorption mechanism for removal of heavy metal ions from aqueous medium. Abundant interlayer anions within LDHs can also be exchanged and the adsorption properties are associated with the types of anions. For example, Wang et al. reported the interlayer nitrate ions in the Mg/Al–NO3 LDHs could exchange with arsenate during adsorption process.16 Kuroda et al. synthesized layered double hydroxide nanoparticles (LDHNPs) using a tripodal ligand of tris(hydroxymethyl)aminomethane, which displayed highly exchangeable capability of CO32− that commonly regarded as impossible.33
In this study, we produced three-dimensional flower-like hierarchical MgAl layered double hydroxides (MgAl-LDHs) with chloride and carbonates ions as interlayer anions via a solvothermal method without any surfactant. These hierarchical MgAl-LDHs had a large surface area (95.7 m2 g−1) and showed excellent adsorption properties for As(V) and F− with maximum capacities of 125.8 and 28.6 mg g−1 under neutral condition, respectively. X-ray diffraction patterns, Fourier transformed infrared spectra, X-ray photoelectron spectroscopy and energy dispersive spectra were used to investigate the adsorption mechanisms of hierarchical MgAl-LDHs for As(V) and F−. The results showed that ion exchange between interlayer anions in hierarchical MgAl-LDHs and As(V) and F− ions was the main adsorption mechanism. Chloride and carbonate ions intercalated in the MgAl-LDHs layers were exchanged with As(V), while only chloride ions were exchanged with F− during adsorption.
Experimental section
Materials
Analytical-grade magnesium chloride hexahydrate (MgCl2·6H2O), aluminum chloride hexahydrate (AlCl3·6H2O), urea, anhydrous methanol were purchased from Beijing Chemicals Co. (Beijing, China). Sodium hydrogen arsenate heptahydrate were purchased from Alfa Aesar. Sodium fluoride (ACS grade >99%) was purchased from Acros Organics. All chemicals were used without further purification. Deionized water used in all of the experiments was prepared using Milli-Q water by Milli-Q system (Millipore, Bedford, MA).Synthesis of MgAl-LDHs
In a typical synthesis, MgCl2·6H2O, AlCl3·6H2O and urea with a molar ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 were dissolved in 60 mL anhydrous methanol at room temperature. After stirred for 30 min, the clear solutions were transferred into a 100 mL autoclave, and then increased the temperature to 150 °C and kept for 6 h. The solid precipitate was collected by centrifugation and washed several times with water and ethanol, respectively. Finally, the product was dried at 80 °C for 12 h, denoted as MgAl-LDHs.
7 were dissolved in 60 mL anhydrous methanol at room temperature. After stirred for 30 min, the clear solutions were transferred into a 100 mL autoclave, and then increased the temperature to 150 °C and kept for 6 h. The solid precipitate was collected by centrifugation and washed several times with water and ethanol, respectively. Finally, the product was dried at 80 °C for 12 h, denoted as MgAl-LDHs.
Characterizations
The microscopic features of the samples were characterized by scanning electron microscopy (SEM, JEOL-6701F), transmission electron microscopy (TEM, JEOL JEM-1011, 100 kV). X-Ray powder diffraction (XRD) patterns were collected on an X-ray diffractometer (Rigaku D/max-2500 diffractometer with Cu-Kα radiation, λ = 0.154056 nm) at 40 kV and 200 mA. The surface area of the products was measured by the Brunauer–Emmett–Teller (BET) method using N2 adsorption and desorption isotherms on an Autosorb-1 analyzer at 78.3 K. X-ray photoelectron spectroscopy (XPS) measurements were performed in a VG Scientific ESCALAB Mark II spectrometer equipped with two ultra-high vacuum (UHV) chambers. Fourier transformed infrared (FTIR) spectra were recorded on a Nicolet Magana-IR 750 spectrometer over a range from 400 to 4000 cm−1. Metal elemental analysis was conducted using Inductively Coupled Plasma Atomic Emission Spectroscopy (ICP-AES, Shimadzu ICPE-9000). The concentration of fluoride was determined by ion chromatography (Dionex ICS-900) equipped with a AS-14A analytical column and a DS5 conductivity detector.Adsorption experiments
Arsenate and fluoride solutions with different concentrations were prepared with Na2HAsO4·7H2O (98%, Alfa) and NaF as the sources, respectively. All the initial solutions were adjusted to pH 7.0 ± 0.1 using HCl (0.2 M). The batch adsorption experiments were carried out in 50 mL polyethylene tubes with 24 mg adsorbents and 30 mL arsenate or fluoride solutions of different concentrations at room temperature. After stirred for 24 h, the solutions were separated from suspensions by centrifugation. Then the upper solutions filtered by 0.22 μm membrane were analyzed by ICP-AES or IC and the solids washed with deionized water twice and heated at 80 °C for 12 h for characterizations. The amount of special adsorbate ions adsorbed per unit weight of the adsorbent at equilibrium (mg g−1), qe was calculated according to the following equation:| qe = (C0 − Ce)V/m |
Results and discussion
Characterizations of flower-like MgAl-LDHs
Fig. 1a shows the typical SEM image of as-prepared MgAl-LDHs. They were composed of many uniform, three-dimensional hierarchical flower-like structures with a diameter of ca. 2–3 μm. The flower-like MgAl-LDHs were built of many nanosheets with the thicknesses of around 50–80 nm and connected to each other to form a highly open structure and no stand-alone plate-like LDHs could be seen. TEM images (Fig. 1b) further confirmed the hierarchical flower-like structures. Nanosheets were self-assembled to form flower-like nanostructures. EDS result (inset in Fig. S1†) revealed the as-prepared MgAl-LDHs composed of Mg, Al, O, C and Cl. Particularly, Cl was detectable and the weight ratio was about 3.55%. The typical X-ray diffraction (XRD) pattern of as-prepared MgAl-LDHs is shown in Fig. 1c. The diffraction peaks was in good agreement with characteristics of LDH-type materials reported in the literatures.34–37 No other diffraction peaks can be found, indicating the purity of as-prepared MgAl-LDHs. The interlayer distance of MgAl-LDHs can be determined as 0.781 nm based on the (003) diffraction peak, which is larger than carbonate type LDHs.17,30,31,38 This implied that there were also other anions co-existed in the interlayer. The FTIR spectrum of as-prepared MgAl-LDHs is shown in Fig. 1d. The strong peak at 3455 cm−1 was attributed to the vibration of hydroxyl groups associated with the interlayer water molecules and hydrogen bonding. The bands around 2956 cm−1 and 1066 cm−1 were assigned to the stretching vibrations of C–H and C–O from the solvent methanol, respectively.39 The strong peak at 1385 cm−1 was attributed to the vibration mode of CO32− in the interlayer of MgAl-LDHs. The bands in the range of 500–1000 cm−1 are attributed to M–O, O–M–O, and M–O–M lattice vibrations (M = Mg and Al). The surface area of flower-like MgAl-LDHs was tested by nitrogen adsorption–desorption (Fig. 2). The BET surface area was calculated to be 95.7 m2 g−1. | ||
| Fig. 1 (a) SEM image, (b) TEM image, (c) XRD pattern and (d) FTIR spectrum of as-prepared MgAl-LDHs. | ||
Based on the above results, we can conclude that chloride ions and carbonates ions were possibly co-exist as the interlayer anions. These anions in the interlayer were very important for the adsorption, which will be discussed in the following section.
Adsorption properties of flower-like MgAl-LDHs
In order to investigate the adsorption process over hierarchical MgAl-LDHs, adsorption isotherms are obtained with initial concentrations of As(V) and F− ranging from 10 to 200 mg L−1. Fig. 3 shows the adsorption isotherms of As(V) and F− ions over MgAl-LDHs. The adsorption data were fitted with both Langmuir and Freundlich models.The Langmuir model is applicable for uniform adsorption processes, where each adsorption site on the surface has identical binding sites and is described as monolayer adsorption.40 Langmuir model can be described as follows:
| qe = qmbCe/(1 + bCe) |
The Freundlich model is an empirical description of adsorption on heterogeneous surface and expressed as:41
| qe = KFCe1/n |
The detailed fitting parameters of the Langmuir and Freundlich models are listed in the Table S1.† It can be seen that the adsorption data of MgAl-LDHs for As(V) was fitted better with Langmuir model than Freundlich model, while opposite for adsorption of F− ions. The maximum adsorption capacities of as-prepared MgAl-LDHs for As(V) and F− were 125.8 and 28.6 mg g−1, respectively. These adsorption capacities were obtained at pH ≈ 7.0, which is similar to the real underground water condition.
Adsorption mechanisms of flower-like MgAl-LDHs for As(V) and F−
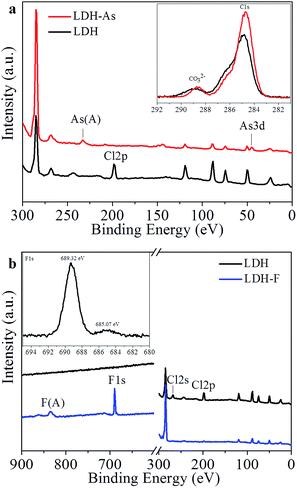
To investigate the detailed adsorption mechanisms for arsenic and fluoride, As(V) and F− saturated flower-like MgAl-LDHs nanostructures were prepared. After adsorption, the solid samples were centrifugated and washed with water for several times, and then dried for characterizations. The adsorption mainly occurred on the surface. Therefore, XPS was used to characterize the surface states of flower-like MgAl-LDHs before and after adsorption of arsenic and fluoride. As shown in Fig. 4a, arsenic signal appeared after adsorption, suggesting As(V) ions were successfully adsorbed on flower-like MgAl-LDHs. However, the peak of Cl 2p at 198 eV disappeared after As(V) adsorption. This suggested that chloride may exchange with As(V). EDS result after As(V) adsorption also showed that As(V) ions were adsorbed and chloride ions were almost not detected (Fig. S2†).Besides, the high resolution C1s spectra of LDHs before and after As(V) adsorption (inset in the Fig. 4a) shows that the intensity of carbonate located at 288.4 eV, which could be assigned to the intercalated CO32− of MgAl-LDHs, decreases significantly after As(V) adsorption, indicating that the partial replacement of interlayer CO32− was also exchanged with As(V). FTIR spectrum of MgAl-LDHs after As(V) adsorption is shown in Fig. S3.† As we can see, the intensity of the band located at 1385 cm−1 of interlayer carbonate was considerably decreased after adsorption of As(V) for MgAl-LDHs. At the same time, a new band of As–O stretching vibration located at 852 cm−1 was detected, further confirming the ion exchange of carbonate with As(V) occurred.42,43
Fig. 4b shows the XPS spectra of MgAl-LDHs before and after fluoride adsorption. Fluoride peak appeared and the peaks at 198 eV (Cl 2p) and 267 eV (Cl 2s) disappeared after fluoride adsorption, indicating ion exchange between chloride and fluoride occurred. EDS result confirmed no detectable chloride was observed in MgAl-LDHs after fluoride adsorption (Fig. S4†). The high resolution of F 1s spectrum is shown in the inset of Fig. 4b. The small peak at 685 eV and the main peak at 689.3 eV can be assigned to physically adsorbed F− on the surface and F− in the lattice of MgAl-LDHs, respectively.19,44,45 This results suggested that fluoride ions mainly intercalated into the layers other than adsorbed on the surface. In addition, no obvious change of C1s spectra was observed of MgAl-LDHs before and after fluoride adsorption, indicating carbonate ions may not participated in the ion exchange during fluoride adsorption.
SEM images of MgAl-LDHs after adsorption of As(V) and F− ions showed that the structures could be maintained (Fig. S5†). XRD patterns of as-prepared MgAl-LDHs after adsorption of As(V) were shown in Fig. 5a. No obvious change of the interlayer spacing of as-prepared flower-like MgAl-LDHs was detected at low concentrations. However, obvious shift of the interlayer spacing to a higher diffraction angle was observed at high concentrations, indicating that As(V) intercalated into the interlayer and expanded the interlayer. While for fluoride adsorption (Fig. 5b), nearly no change can be seen with full range of concentrations. This was because only chloride ions were exchanged with fluoride ions and nearly the same size of them.
 | ||
| Fig. 5 XRD patterns of MgAl-LDHs after adsorption of (a) As(V) and (b) F− with different initial concentrations. | ||
Based on the above results, ion exchange between interlayer anions in hierarchical MgAl-LDHs and As(V) and F− ions in solution was the main adsorption mechanism. Chloride and carbonate ions intercalated in the MgAl-LDHs layers were exchanged with As(V), while only chloride ions were exchanged with F− during adsorption.
In general, MgAl-LDHs have greater affinity for multivalent inorganic anions compared with monovalent inorganic anions. The affinity has been reported to decrease in the order CO32− > SO42− > F− > Cl− > NO3−,46 which is consistent with our experimental results. Based on this order, the fact in this work that chlorides, carbonates intercalated in the LDHs layers were exchanged with As(V) while only chlorides were exchanged with fluorides during adsorption could be explained. The fact that only Cl− was involved in F− adsorption, while both Cl− and CO32− were involved in As(V) adsorption is worth investigation, as it was related to the distribution of anions in the clay layer, and how guest anions can enter the interlayer space.
Conclusions
Three-dimensional hierarchical MgAl layered double hydroxides (MgAl-LDHs) with carbonate and chloride ions as the interlayer anions were produced by a solvothermal method without adding surfactant. These hierarchical MgAl-LDHs showed excellent adsorption properties for As(V) and F− with maximum capacities of 125.8 and 28.6 mg g−1 under neutral condition, respectively. Ion exchange between interlayer anions in hierarchical MgAl-LDHs and As(V) and F− ions was the main adsorption mechanism, which was very different from the structure reconstruction mechanism of calcined MgAl-LDHs. Chloride and carbonate ions intercalated in the as-prepared MgAl-LDHs layers were exchanged with As(V), while only chloride ions were exchanged with F− during adsorption.Acknowledgements
We thank the financial support from the National Basic Research Program of China (2011CB933700, 2012BAJ25B08), the National Natural Science Youth Foundation of China (NSFC 51402305) and the Chinese Academy of Sciences (XDA09030200, GJHZ1224, KJCX2-YW-N41).References
- D. Mohan and C. U. Pittman Jr, J. Hazard. Mater., 2007, 142, 1 CrossRef CAS PubMed.
- X. Zhuang, Y. Wan and C. M. Feng, et al., Chem. Mater., 2009, 21, 706 CrossRef CAS.
- A. Bhatnagar, E. Kumar and M. Sillanpää, Chem. Eng. J., 2011, 171, 811 CrossRef CAS PubMed.
- G. X. Zhao, J. X. Li and X. M. Ren, et al., Environ. Sci. Technol., 2011, 45, 10454 CrossRef CAS PubMed.
- H. W. Liang, X. Cao and W. J. Zhang, et al., Adv. Funct. Mater., 2011, 21, 3851 CrossRef CAS.
- W. Li, C. Y. Cao and L. Y. Wu, et al., J. Hazard. Mater., 2011, 198, 143 CrossRef CAS PubMed.
- H. W. Liang, Q. F. Guan and L. F. Chen, et al., Angew. Chem., Int. Ed., 2012, 51, 5101 CrossRef CAS PubMed.
- B. Wang, H. B. Wu and L. Yu, et al., Adv. Mater., 2012, 24, 1111 CrossRef CAS PubMed.
- C. Y. Cao, J. Qu and W. S. Yan, et al., Langmuir, 2012, 28, 4573 CrossRef CAS PubMed.
- Z. X. Wu, W. Li and P. A. Webley, et al., Adv. Mater., 2012, 24, 485 CrossRef CAS PubMed.
- J. Qu, C. Y. Cao and W. Li, et al., J. Mater. Chem., 2012, 22(33), 17222 RSC.
- H. Chen, J. X. Li and X. L. Wu, et al., Ind. Eng. Chem. Res., 2014, 53, 16051 CrossRef CAS.
- H. J. Yu, Y. Y. Lv and K. Y. Ma, et al., J. Colloid Interface Sci., 2014, 428, 251 CrossRef CAS PubMed.
- X. M. Zhang, J. Y. Liu and S. J. Kelly, et al., J. Mater. Chem. A, 2014, 2, 11759 CAS.
- K. Mandel, A. Drenkova-Tuhtan and F. Hutter, et al., J. Mater. Chem. A, 2013, 1, 1840 CAS.
- S. L. Wang, C. H. Liu and M. K. Wang, et al., Appl. Clay Sci., 2009, 43, 79 CrossRef CAS PubMed.
- T. Wen, X. L. Wu and X. L. Tan, et al., ACS Appl. Mater. Interfaces, 2013, 5, 3304 CAS.
- Y. F. Xu, Y. C. Dai and J. Z. Zhou, et al., J. Mater. Chem., 2010, 20, 4684 RSC.
- J. B. Zhou, Y. Cheng and J. G. Yu, et al., J. Mater. Chem., 2011, 21, 19353 RSC.
- X. L. Wu, X. L. Tan and S. T. Yang, et al., Water Res., 2013, 47, 4159 CrossRef CAS PubMed.
- L. C. Tan, Y. L. Wang and Q. Liu, et al., Chem. Eng. J., 2015, 259, 752 CrossRef CAS PubMed.
- P. Gunawan and R. Xu, Chem. Mater., 2009, 21, 781 CrossRef CAS.
- L. S. Zhong, J. S. Hu and A. M. Cao, et al., Chem. Mater., 2007, 19, 1648 CrossRef CAS.
- J. S. Hu, L. S. Zhong and W. G. Song, et al., Adv. Mater., 2008, 20, 2977 CrossRef CAS.
- H. Li, W. Li and Y. J. Zhang, et al., J. Mater. Chem., 2011, 21, 7878 RSC.
- J. Qu, C. Y. Cao and Y. L. Hong, et al., J. Mater. Chem., 2012, 22, 3562 RSC.
- C. Y. Cao, J. Qu and F. Wei, et al., ACS Appl. Mater. Interfaces, 2012, 4, 4283 CAS.
- S. W. Zhang, J. X. Li and T. Wen, et al., RSC Adv., 2013, 3, 2754 RSC.
- C. P. Chen, P. H. Wang and T. T. Lim, et al., J. Mater. Chem. A, 2013, 1, 3877 CAS.
- L. Lv, J. He and M. Wei, et al., J. Hazard. Mater., 2006, 133, 119 CrossRef CAS PubMed.
- X. Y. Yu, T. Luo and Y. Jia, et al., Nanoscale, 2012, 4, 3466 RSC.
- K. H. Goh, T. T. Lim and Z. L. Dong, Water Res., 2008, 42, 1343 CrossRef CAS PubMed.
- Y. Kuroda, Y. Miyamoto and M. Hibino, et al., Chem. Mater., 2013, 25, 2291 CrossRef CAS.
- E. Gardner, K. M. Huntoon and T. J. Pinnavaia, Adv. Mater., 2001, 13, 1263 CrossRef CAS.
- B. Li and J. He, J. Phys. Chem. C, 2008, 112, 10909 CAS.
- J. G. Wang, Y. Wei and J. Yu, Appl. Clay Sci., 2013, 72, 37 CrossRef CAS PubMed.
- Z. P. Xu and G. Q. Lu, Chem. Mater., 2005, 17, 1055 CrossRef CAS.
- A. G. Caporale, M. Pigna and J. J. Dynes, et al., J. Hazard. Mater., 2011, 198, 291 CrossRef CAS PubMed.
- J. A. Gursky, S. D. Blough and C. Luna, et al., J. Am. Chem. Soc., 2006, 128, 8376 CrossRef CAS PubMed.
- I. Langmuir, J. Am. Chem. Soc., 1916, 38, 2221 CrossRef CAS.
- H. Freundlich, Z. Phys. Chem., 1906, 57, 385 CAS.
- N. Mahanta and J. P. Chen, J. Mater. Chem. A, 2013, 1, 8636 CAS.
- S. V. Prasanna and P. V. Kamath, J. Colloid Interface Sci., 2009, 331, 439 CrossRef CAS PubMed.
- J. G. Yu, W. G. Wang and B. Cheng, et al., J. Phys. Chem. C, 2009, 113, 6743 CAS.
- Y. L. Tang, X. H. Guan and J. M. Wang, et al., J. Hazard. Mater., 2009, 171, 774 CrossRef CAS PubMed.
- S. Miyata, Clay Clay Miner., 1983, 31, 305 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: EDS and FTIR spectra of MgAl-LDHs before and after adsorption of As(V) and F−, and table of summary of the Langmuir and Freundlich isotherm model parameters for the As(V)/F− uptake capacity on LDHs. See DOI: 10.1039/c4ra15160g |
| This journal is © The Royal Society of Chemistry 2015 |