Hierarchical CdS nanostructure by Lawesson's reagent and its enhanced photocatalytic hydrogen production†
Vikram U. Pandita,
Sudhir S. Arbuja,
Ranjit R. Hawaldara,
Pradnya V. Kshirsagara,
Amarsinh J. Deshmukhb,
Jalindar D. Ambekara,
Uttam P. Mulika,
Suresh W. Gosavic and
Bharat B. Kale*a
aCentre for Materials for Electronic Technology (C-MET), Department of Electronics and Information Technology (DeitY), Govt of India, Panchawati, Off Pashan Road, Pune 411008, India. E-mail: kbbb1@yahoo.com; bbkale@cmet.gov.in; Fax: +91-20-25898085; Tel: +91-20-25898390
bNational Chemical Laboratory (NCL), Dr. HomiBhabha Road, Pune-411008, India
cDepartment of Physics, University of Pune, Pune-411007, India
First published on 20th January 2015
Abstract
Lawesson's reagent (LR) has been effectively exploited for the synthesis of hierarchical architectures of cadmium sulphide (CdS) nanostructures for the first time. The X-ray diffractograms of the as synthesised CdS nanostructures confirm the formation of hexagonal CdS. The broadness of the XRD peaks clearly indicates the nanocrystalline nature of CdS with average crystallite size of 4 nm. A FESEM study revealed the formation of hierarchical nanostructures, whereas a TEM study showed that the hierarchical arrangement is composed of nanosized CdS particles. A band-gap i.e. 2.4 eV was derived from diffuse reflectance spectroscopy. The photoluminescence spectrum showed an emission peak at 535 and 568 nm which can be attributed to band-edge emission and surface emissions or possible metal vacancies, respectively. Considering the band-gap within the visible region, the photocatalytic hydrogen evolution performance of these CdS nanostructures was performed under visible light irradiation from hydrogen sulphide and water, respectively. Utmost hydrogen evolution i.e. 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 136 μmol h−1 g−1 and 2065 μmol h−1 g−1 was observed over a naked CdS nanostructure via H2S and water decomposition, respectively. The amount of hydrogen obtained by H2S splitting is much higher as compared to earlier reports.
136 μmol h−1 g−1 and 2065 μmol h−1 g−1 was observed over a naked CdS nanostructure via H2S and water decomposition, respectively. The amount of hydrogen obtained by H2S splitting is much higher as compared to earlier reports.
1. Introduction
Over the last few decades, nanostructured materials have been at the centre of attention owing to their fascinating size-dependent optical, electronic, thermal, mechanical, chemical, and physical properties.1–3 These nanomaterial display unique properties not only from their bulk counterparts but also from those of the atomic or molecular precursors from which they are synthesized. For the fabrication of next-generation optoelectronic devices and for fulfilling energy demands the use of nanoscale semiconductor nanostructures is imperative.4,5 Of late, lasers, sensors and field emitters using nanomaterials have been demonstrated by many researchers. Instead of all the efforts to date, there exists a quest to find better, cheaper and efficient nanomaterials.6,7In this context, cadmium sulphide with a band gap 2.4 eV is a promising candidate owing to its wide ranging applications in nonlinear optical devices,8 flat-panel displays, light-emitting diodes,9 lasers,10,11 logic gates, and transistors, wide application in telecommunications, data storage, and near-field optical lithography.12 Hence, a lot of efforts have been concentrated to study the effect of manipulation of size, shape and crystal structure of CdS materials on their optoelectronic properties.13 Studies have also been devoted for the synthesis and characterization of various nanostructures, such as nanotubes, nanowires, nanorods, and nanobelts.14,15 The ideal band-gap of CdS in bulk as well as nano form makes it an attractive semiconductor for photocatalytic applications like hydrogen sulphide (H2S) and water splitting. A large quantity of H2S is produced in oil refineries which possess a serious environmental threat. Once produced, it remains for many years in environment thereby causing pollution.16 Till date, only few techniques have been effectively used for treating this H2S. Of all the H2S produced globally, a negligible amount is utilized to produce sulphur by Claus process for agricultural and pharmaceutical industries.17–19 This process is not eco-friendly as it produces large amount of waste and is also energy intensive. Oil refineries or natural gas extraction satisfy the basic demand for fuel of the uncontrollably growing global population and hence their operations cannot be terminated. Therefore, there is an immediate demand to convert environmentally hazardous side products like H2S into viable fuel sources like H2.20,21
Hydrogen is an ideal fuel for future owing to its clean and renewable nature. Hydrogen can be produced by gasification from biomass, methane reforming, through the electrolysis of H2O or H2S and photocatalytic cleavage of H2S or H2O.16,22,23 Of all the processes, the photocatalytic processes using solar energy for hydrogen production seems to be the most environmentally safe and less energy intensive methodology as solar energy is free of cost and abundantly available.
Among all the available catalysts, CdS is a promising photocatalyst for H2S as well as H2O splitting due to suitable position of the conduction band edge that is more negative than the reduction potential of H+/H2.27 Many approaches for improving the hydrogen production rate and photo-stability of the photocatalyst have been investigated/are under investigation.28 For example, CdS loaded with MoS2 co-loaded with Pt and PdS, incorporation of CdS particles into the mesopores of Ti-MCM-41 and introduction of graphene nanosheets into CdS have been used.29–32 In general, there are different factors that control the H2 production rate on CdS photocatalyst, such as morphology, particle size, phase structure and crystalline nature.33–37 A variety of approaches have been proposed to enhance the photocatalytic activity of CdS photocatalyst, including the synthesis of CdS nano material's with various morphologies (nanowires, nanorods, nanospheres, nanosheets etc.) and quantum-sized CdS.38–46 In spite of recent advances in synthesis of nanomaterial, the production of CdS nanostructures still requires an expensive template and harsh conditions, which limit large-scale production or commercialization of the process.47–49
With all these clues in mind and our experience17–25 in H2S splitting for H2 production using UV-visible light, we decided to explore the efficacy of ultra-small CdS nanostructures produced by using an inexpensive and simple procedure with Lawesson's reagent as a sulphur source for visible-light driven photocatalytic hydrogen generation from H2S and H2O. The formation mechanism and optical properties have also been discussed.
2. Experimental
2.1. Synthesis of CdS flowers
In a dry 100 mL beaker, 2 g of cadmium nitrate was dissolved in small amount of water and in another 100 mL beaker 0.70 g of Lawesson's reagent (97%, Spectrochem) was dissolved in chloroform. Both the solutions were transferred to 150 mL teflon coated hydrothermal reactor and kept for 90 °C for 15 h. The yellow solids are filtered and washed with water and copious amount of chloroform to remove unreacted starting materials.2.2. Characterization
Cadmium sulphide nanostructures thus synthesized were characterized by X-ray diffraction (Model-D8, Advance, Bruker AXS). The samples were also characterized with field emission scanning electron microscope (FESEM) and transmission electron microscopy (TEM, model Philips, EM-CM-12) to determine the morphology and particle size respectively. The optical properties were recorded using UV-visible-near infrared (UV-Vis-NIR) spectrophotometer (Perkin Elmer lambda-950 spectrometer). Photoluminescence spectra were recorded by the photoluminescence spectrometer Perkin-Elmer-LS-55. The formation of side products during the reaction was confirmed by 1H NMR (Varian-NMR-Mercury 300). The quantification of H2 and O2 was carried out using Gas Chromatograph (Model Shimadzu GC-14B, MS-5 Å column, TCD, Ar carrier).2.3. Photocatalytic activity measurement
3. Results and discussion
3.1. Materials characterization
Furthermore, to obtain well-defined crystalline CdS, the reaction was performed at 15 h. X-ray powder diffraction (XRD) pattern (Fig. 1) for this sample displays peaks at 2θ = 24.8°, 26°, 27.8°, 44°, and 52.5° corresponding to (100), (002), (101), (110) and (112) crystal planes of hexagonal CdS (JCPDS no. 41-1049), respectively. The broad XRD peaks indicate the formation of nano-structured CdS. The average crystallite size calculated using Scherrer's formula is found to be 4 nm.
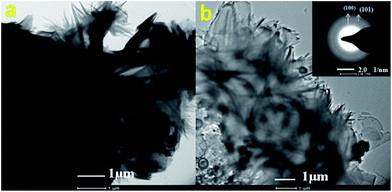 | ||
| Fig. 3 TEM micrograph of CdS nanoparticles and SAED image (inset of Fig. 3b). | ||
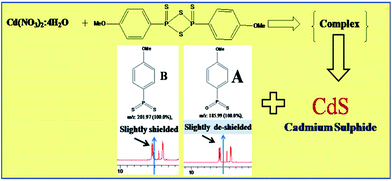
Cadmium nitrate (aqueous), reacts with LR compound which on dissolution with chloroform, dissociate to form a monomer (B) with well-known mechanism (see ESI, Scheme S1†).52–54 The monomer (B) forms a complex with cadmium as shown in the Scheme 1. Further, this complex upon decomposition under hydrothermal conditions forms cadmium sulphide and side products A and unreacted starting monomer B. The as formed CdS using Lawesson's reagent was exclusively characterized by XRD and TEM described above. The side products A and B formed in this reaction was analysed and confirmed by using 1H NMR (ESI, S3 and S4† respectively) and mass spectroscopy. Both the side products are having nearly same 1H NMR spectrum but only in case of the A, the aromatic protons are slightly more deshielded than the B due to electro negativity of oxygen which can be clearly seen in the schematic of the mechanism (Scheme 1). The replacement of sulphur by the oxygen from monomer B is a further evidence for the formation of cadmium sulphide.
During hydrothermal reaction, the intermediate complex slowly decomposed and tiny nuclei of CdS were formed (see Scheme 2A). Initially, tiny nuclei of CdS are produced in the supersaturated solution and further growth of nanoparticles take place with time (Scheme 2b). Under prolonged hydrothermal conditions, the CdS nuclei were grown to form CdS nanocrystals. The newly formed CdS nanoparticles are spontaneously aggregated to minimize their surface energy. These nanoparticles further grow anisotropically along the 2D direction results formation of thin nanopetals like morphology (Scheme 2b and c).
Initially, these nanopetals are transformed into curving nanopetals due to the surface strain created by vapor pressure of solvent at hydrothermal condition (Scheme 2c). At prolonged hydrothermal reaction time, further growth is hindered due to reduction in the precursor concentration. Hence, with prolong reaction time, these curving nanopetals are self-assembled and form a 3D hierarchical nanostructure i.e. flower like structure (depicted in Scheme 2d). Additionally, the use of chloroform as a solvent for this reaction is aiding the formation of CdS nanostructures. Generally, the dielectric constant of solvents governs the morphology of the product. The use of ethylenediamine gives rod like morphology which is well known for one dimensional growth due to its pristine structure whereas ethylene glycol produce spherical particles. It is quite well known that the use of different solvents with different dielectric constant produces different morphologies.52 The BET surface area of CdS nanostructure was observed to be 54 m2 g−1.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 136 μmol h−1 g−1 which is much higher than earlier reports for the same photocatalyst (ESI, Table S1†).17–26 It also showed good stability under the same experimental conditions.
136 μmol h−1 g−1 which is much higher than earlier reports for the same photocatalyst (ESI, Table S1†).17–26 It also showed good stability under the same experimental conditions.
In 0.25 M KOH solution (pH 12.5), the weak diprotic acid H2S (two pKa values are 7.0 and 11.96) dissociates and maintains equilibrium with hydrosulphide HS− ions (1). The CdS absorbs light and generates electron–hole pairs (2). The photo-generated valence band hole (h+VB) upon band gap excitation of CdS nanopowder oxidizes the HS− ion to disulphide ion (S22−), liberating a proton from the HS− ion (3). The conduction band electron (e−CB) from CdS photocatalyst reduces protons to produce molecular hydrogen (4).
| H2S + OH− ↔ HS− + H2O | (1) |
| Semiconductor CdS ↔ h+VB + e−CB | (2) |
| Oxidation: 2HS− + 2h+VB ↔ S22− + 2H+ | (3) |
| Reduction: 2H+ + 2e−CB ↔ H2 | (4) |
The efficiency of our photocatalyst was confirmed by the experiment which showed that in absence of photocatalyst as well as in dark condition, evolution of hydrogen gas was not observed from 0.25 M KOH solution flushed with H2S gas under the visible light irradiation. Also, we carried out the recycle study of CdS nanostructures and found almost same hydrogen production even after three cycles (see ESI, Table S2†).
Hence, under the given experimental conditions, the presence of a sacrificial agent, an electron sink in the form of Pt on the surface and the size of the photocatalyst particles have perceptible influence on the photocatalytic hydrogen production. Among the studied sacrificial reagents, benzyl alcohol gave highest H2 gas evolution around 2065 μmol h−1 g−1.28 In presence of Na2S and Na2SO3 rate of H2 production is 1060 μmol h−1 g−1 whereas, in presence of methanol lower rate of H2 generation was observed (223 μmol h−1 g−1).
We also performed the H2 generation in absence of Pt as a co-catalyst and found 103 μmol h−1 g−1 of H2 evolution. The TEM images of Pt-loaded CdS are also depicted in ESI (Fig. S4†). In absence of Pt the formed electron–hole pair recombines rather being getting separated which result lower H2 evolution.
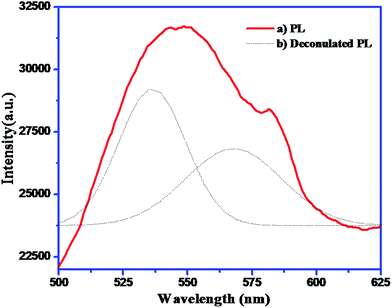
Considering the earlier reports for H2S splitting, the flowers of CdS gave enhanced photocatalytic activity due to high aspect ratio. If we see the FESEM carefully, each petal has average size is 0.5 (W) × 1.5 (L) μm. Each petal has an average thickness around 40 to 50 nm. The flower is composed of such individual petals which ultimately have higher aspect ratio. All flowers are observed to be puffy in nature and greatly expose to the light. Hence, maximum surface is exposed to the light which enhances overall photo-absorption which ultimately increases photo-generated charge carriers, significantly.17 The photoluminescence study clearly indicates broad emission peak at 568 nm due to defects created by metal vacancies. This defects may be acting as a mid-trap vacancies which decreases electron and hole recombination by trapping the holes. Additionally, due to thin petals (plate structure), transport of electron to the surface also get enhanced. Since, it is well known that free electrons are responsible for water and H2S splitting; the enhancement in hydrogen evolution is quite justifiable. Additionally, the surface area observed to be fairly larger which is also responsible for good activity.
The stability of the photocatalyst is already confirmed by performing recycle experiments. The chemistry of photo-corrosion is well understood in case of CdS for water splitting. However, in case of H2S splitting reaction in presence of sacrificial reagents, the photo-corrosion process is hindered and ultimately the formation of Cd ions also negligible. There is a continuous supply of H2S during reaction which favours the CdS formation and suppresses the formation of cadmium ion or any other cadmium compound. In case of H2 production from H2S, Cd ions (if formed) may be reacting with the S ions which are already available as per reaction (1) in alkaline medium. Even if the Cd ion or CdO formed we can easily convert it into CdS by treating it with Lawesson's reagent. Hence, in technological point of view, catalyst regeneration is very easy in the present system. In view of the above, there will not be any leaching and disposal of cadmium which avoids the environmental issue. The large scale production of CdS nanostructures using the Lawesson's reagent is possible. With the use of sophisticated equipment's (Parr reactor) the CdS with same physico-chemical properties can be reproducible.
4. Conclusions
In summary, we have architectured hierarchical CdS nanostructures i.e. carnation flower like morphology using simple hydrothermal method. We have used Lawesson's reagent as a sulphur source as well as a capping agent for the first time. Considering the unique morphology, the possible formation mechanism has been furnished. The CdS carnation flowers showed highest photocatalytic H2 evolution i.e. 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 136 μmol h−1 g−1 for H2S splitting which is much higher than the earlier reports on CdS nanoparticles/bulk for the same reaction. The CdS nano carnation flowers also showed good H2 evolution i.e. 2065 μmol h−1 g−1 from water. The rate of H2 generation was highly dependent on type of sacrificial reagents. The enhanced photocatalytic activity is due to puffy flower like morphology which ultimately enhances photo-absorption and photo-generated charge carriers. More significantly, the catalyst has shown very stable activity after recycles. Such kinds of nanostructures are attractive candidates for solar cell and other optoelectronic devices. The present investigation will be greatly helpful for the synthesis of other hierarchically nanostructured metal chalcogenides from Lawesson's reagent.
136 μmol h−1 g−1 for H2S splitting which is much higher than the earlier reports on CdS nanoparticles/bulk for the same reaction. The CdS nano carnation flowers also showed good H2 evolution i.e. 2065 μmol h−1 g−1 from water. The rate of H2 generation was highly dependent on type of sacrificial reagents. The enhanced photocatalytic activity is due to puffy flower like morphology which ultimately enhances photo-absorption and photo-generated charge carriers. More significantly, the catalyst has shown very stable activity after recycles. Such kinds of nanostructures are attractive candidates for solar cell and other optoelectronic devices. The present investigation will be greatly helpful for the synthesis of other hierarchically nanostructured metal chalcogenides from Lawesson's reagent.
Acknowledgements
Authors are grateful to C-MET and DeitY, New Delhi for providing the facilities. Vikram Pandit would like to thank the Council of Scientific and Industrial Research, CSIR, New Delhi, India for financial support.References
- H. M. Chen, C. K. Chen, R. Liu, L. Zhang, J. Zhang and D. P. Wilkinson, Chem. Soc. Rev., 2012, 41, 5654–5671 RSC.
- X. Chen, C. Li, M. Gratzel, R. Kosteckid and S. S. Mao, Chem. Soc. Rev., 2012, 41, 7909–7937 RSC.
- F. E. Osterloh, Chem. Soc. Rev., 2013, 42, 2294–2320 RSC.
- P. Zhang, J. Zhang and J. Gong, Chem. Soc. Rev., 2014, 43, 4395–4422 RSC.
- J. Ran, J. Zhang, J. Yu, M. Jaroniecc and S. Z. Qiao, Chem. Soc. Rev., 2014, 43, 7787–7812 RSC.
- T. Hisatomi, J. Kubota and K. Domen, Chem. Soc. Rev., 2014, 43, 7520–7535 RSC.
- H. You, S. Yang, B. Dinga and H. Yang, Chem. Soc. Rev., 2013, 42, 2880–2904 RSC.
- H. Kind, H. Yan, B. Messer, M. Law and P. D. Yang, Adv. Mater., 2002, 14, 158–160 CrossRef CAS.
- X. S. Fang, Y. Bando, U. K. Gautam, C. H. Ye and D. Golberg, J. Mater. Chem., 2008, 18, 509–522 RSC.
- B. D. Liu, T. Y. Zhai, T. Sekiguchi, Y. Koide and D. Golberg, Adv. Mater., 2009, 21, 2034–2039 CrossRef.
- Z. B. He, J. S. Jie, W. J. Zhang, W. F. Zhang, L. B. Luo, X. Fan, G. D. Yuan, I. Bello and S. T. Lee, Small, 2009, 5, 345–350 CrossRef CAS PubMed.
- M. H. Huang, S. Mao, H. Feick, H. Yan, Y. Wu, H. Kind, E. Weber, R. Russo and P. D. Yang, Science, 2001, 92, 1897–1899 CrossRef PubMed.
- T. Y. Zhai, Z. J. Gu, H. Z. Zhong, Y. Dong, Y. Ma, H. B. Fu, Y. F. Li and J. N. Yao, Cryst. Growth Des., 2007, 7, 448–491 Search PubMed.
- G. Z. Shen and C. J. Lee, Cryst. Growth Des., 2005, 5, 1085–1089 CAS.
- X. F. Duan, C. M. Niu, V. Sahi, J. Chen, J. W. Parce, S. Empedocles and J. L. Goldman, Nature, 2003, 425, 274–278 CrossRef CAS PubMed.
- M. Gratzel, Nature, 2001, 414, 338–344 CrossRef CAS PubMed.
- B. B. Kale, J. O. Baeg, S. M. Lee, H. Chang, S. J. Moon and C. W. Lee, Adv. Funct. Mater., 2006, 16, 1349–1354 CrossRef CAS.
- S. K. Apte, S. N. Garaje, G. P. Mane, A. Vinu, S. D. Naik, D. P. Amalnerkar and B. B. Kale, Small, 2011, 7(7), 957–964 CrossRef CAS PubMed.
- S. K. Apte, S. N. Garaje, S. D. Naik, R. P. Waichal, J. O. Baeg and B. B. Kale, Nanoscale, 2014, 6, 908–915 RSC.
- V. U. Pandit, S. S. Arbuj, U. P. Mulik and B. B. Kale, Environ. Sci. Technol., 2014, 48(7), 4178–4183 CrossRef CAS PubMed.
- S. K. Apte, S. N. Garaje, S. D. Naik, R. P. Waichal and B. B. Kale, Green Chem., 2013, 15, 3459–3467 RSC.
- S. K. Apte, S. N. Garaje, M. Valant and B. B. Kale, Green Chem., 2012, 14, 1455–1462 RSC.
- N. S. Chaudhari, A. P. Bhirud, R. S. Sonawane, L. K. Nikam, S. S. Warule, V. H. Rane and B. B. Kale, Green Chem., 2011, 13, 2500–2506 RSC.
- S. K. Apte, S. N. Garaje, S. S. Arbuj, B. B. Kale, J. O. Baeg, U. P. Mulik, S. D. Naik, D. P. Amalnerkar and S. W. Gosavi, J. Mater. Chem., 2011, 21, 19241–19248 RSC.
- B. B. Kale, J. O. Baeg, S. K. Apte, R. S. Sonawane, S. D. Naik and K. R. Patil, J. Mater. Chem., 2007, 17, 4297–4303 RSC.
- N. S. Chaudhari, S. S. Warule, S. A. Dhanmane, M. V. Kulkarni, M. Valant and B. B. Kale, Nanoscale, 2013, 5, 9383–9390 RSC.
- T. D. Vu, F. Mighri, A. Ajji and T. Do, Ind. Eng. Chem. Res., 2014, 53, 3888–3897 CrossRef CAS.
- S. R. Lingampalli, U. K. Gautam and C. N. R. Rao, Energy Environ. Sci., 2013, 6, 3589–3594 CAS.
- W. Li, S. Xie, M. Li, X. Ouyang, G. Cui, X. Lu and Y. Tong, J. Mater. Chem. A, 2013, 1, 4190–4193 CAS.
- Y. Xu, W. Zhao, R. Xu, Y. Shi and B. Zhang, Chem. Commun., 2013, 49, 9803–9805 RSC.
- T. Xuan, S. Wang, X. Wang, J. Liu, J. Chen, H. Li, L. Pana and Z. Sun, Chem. Commun., 2013, 49, 9045–9047 RSC.
- R. Peng, C. Wu, J. Baltrusaitis, N. Dimitrijevic, T. Rajh and R. Koodali, Chem. Commun., 2013, 49, 3221–3223 RSC.
- J. He, Z. Yan, J. Wang, J. Xie, L. Jiang, Y. Shi, F. Yuan, F. Yu and Y. Sun, Chem. Commun., 2013, 49, 6761–6763 RSC.
- A. B. Panda, G. Glaspell and M. S. Shall, J. Am. Chem. Soc., 2006, 128, 2790–2791 CrossRef CAS PubMed.
- J. Zhang, S. Z. Qiao, L. Qi and J. Yu, Phys. Chem. Chem. Phys., 2013, 15, 12088–12094 RSC.
- L. Mao, Y. Wang, Y. Zhong, J. Ning and Y. Hu, J. Mater. Chem. A, 2013, 1, 8101–8104 CAS.
- Z. B. Yu, Y. P. Xie, G. Liu, G. Q. Lu, X. L. Ma and H. M. Cheng, J. Mater. Chem. A, 2013, 1, 2773–2776 CAS.
- X. Zong, J. Han, G. Ma, H. Yan, G. Wu and C. Li, J. Phys. Chem. C, 2011, 115, 12202–12208 CAS.
- D. Barpuzary, Z. Khan, N. Vinothkumar, M. De and M. Qureshi, J. Phys. Chem. C, 2012, 116, 150–156 CAS.
- J. Jin, J. Yu, G. Liu and P. K. Wong, J. Mater. Chem. A, 2013, 1, 10927–10934 CAS.
- Y. Wang, Y. Wang and R. Xu, J. Phys. Chem. C, 2013, 117, 783–790 CAS.
- D. Lang, Q. Xiang, G. Qiu, X. Feng and F. Liu, Dalton Trans., 2014, 43, 7245–7253 RSC.
- X. Wang, L. Yinb and G. Liu, Chem. Commun., 2014, 50, 3460–3463 RSC.
- J. Yu, J. Jin, B. Chenga and M. Jaroniec, J. Mater. Chem. A, 2014, 2, 3407–3416 CAS.
- Y. Min, G. Q. He, Q. J. Xu and Y. C. Chen, J. Mater. Chem. A, 2014, 2, 2578–2584 CAS.
- Y. Peng, Z. Guo, J. Yang, D. Wang and W. Yuan, J. Mater. Chem. A, 2014, 2, 6296–6300 CAS.
- S. Liu, M. Q. Yangab and Y. J. Xu, J. Mater. Chem. A, 2014, 2, 430–440 CAS.
- F. Wang, Y. Wang, X. Zhan, M. Safdar, J. Gong and J. He, CrystEngComm, 2014, 16, 1389–1394 RSC.
- Y. Shi, K. Zhou, B. Wang, S. Jiang, X. Qian, Z. Gui, R. K. K. Yuen and Y. Hu, J. Mater. Chem. A, 2014, 2, 535–544 CAS.
- J. K. Vaishnav, S. S. Arbuj, S. B. Rane and D. P. Amalnerkar, RSC Adv., 2014, 4, 47637–47642 RSC.
- H. Weller, Angew. Chem., Int. Ed. Engl., 1993, 32, 41–53 CrossRef.
- H. Z. Lecher, R. A. Greenwood, K. C. Whitehouse and T. H. Chao, J. Am. Chem. Soc., 1956, 78(19), 5018–5022 CrossRef CAS.
- T. J. Curphey, J. Org. Chem., 2002, 67(18), 6461–6473 CrossRef CAS PubMed.
- T. Ozturk, E. Ertas and O. Mert, Chem. Rev., 2007, 107(11), 5210–5278 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: (Fig. S1) Schematic representation of H2S splitting setup, (Scheme S1) mechanism for the formation of monomer (B), (Fig. S2) TEM images of CdS nanostructures and Pt–CdS, (Fig. S3 and S4) 1H NMR spectra of side products A and B respectively. Comparison of H2 production with earlier reports (Table S1) and the recycle study of H2S generation (Table S2). See DOI: 10.1039/c4ra15138k |
| This journal is © The Royal Society of Chemistry 2015 |