Potential in vitro and in vivo colon specific anticancer activity in a HCT-116 xenograft nude mice model: targeted delivery using enteric coated folate modified nanoparticles
Abstract
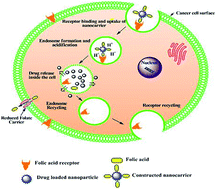
The aim of this study was to develop a drug delivery system for specific targeting in colon cancer treatment. We have developed a Eudragit S-100 (ES) coated folic acid (FA) conjugated gliadin (Gd) delivery system for the effective targeting of overexpressed folate receptors (FRs) in colon cancer. The FA conjugate with Gd (FA–Gd) was synthesized and characterized using FTIR and 1H NMR, and this developed conjugate was used to prepare curcumin (CU) loaded nanoparticles (NPs) by a desolvation method. FA–CU–GdNPs were further coated with ES and ES–FA–CU–GdNPs were obtained. The ES–FA–CU–GdNPs were also capable of inducing cell caspase dependent apoptosis in Caco-2 cell lines and exhibited DNA intercalating activity. In therapeutic experiments the ES–FA–CU–GdNPs were administered orally to HCT-116 tumor-bearing nude mice. In vivo bio-distribution data showed that ES–FA–CU–GdNPs had delivered the maximum amount of NPs to the colon and tumor after 12 hours, reflecting its targeting potential for the colon and tumor. A gamma scintigraphy study suggested that ES–FA–CU–GdNPs remain intact at low pH and released NPs slowly at pH 7.4 in the colon. This study provides evidence that ES–FA–CU–GdNPs hold the promise to address overexpressed FRs in colorectal cancer and were found to be safe for oral administration for a prolonged duration.

 Please wait while we load your content...
Please wait while we load your content...