Hierarchical porous carbon based on the self-templating structure of rice husk for high-performance supercapacitors†
Dechen Liua,
Wenli Zhanga,
Haibo Lin*ab,
Yang Lia,
Haiyan Lu*a and
Yan Wanga
aCollege of Chemistry, Jilin University, Changchun 130012, China. E-mail: lhb910@jlu.edu.cn; luhy@jlu.edu.cn; Fax: +86 431 85155189; Tel: +86 431 85155189
bKey Laboratory of Physics and Technology for Advanced Batteries of Ministry of Education, Jilin University, Changchun, 130012, China
First published on 10th February 2015
Abstract
Using the self-templating structure of rice husk (RH), hierarchical porous carbon with high surface area was prepared by carbonization, NaOH-leaching and KOH activation. The formation processes of the hierarchical porous structure were discussed in detail, where the silica in RH plays a crucial role. The rice husk-based hierarchical porous carbon (RHHPC) with a 3D porous structure, was composed of macrochannels, mesopores and micropores both on its surface and inner channels. The RHHPC exhibited a surface area as high as 2804 m2 g−1 and a high-level of oxygen-containing groups (with an oxygen content of 9.74 at%). The high surface area, large amount of oxygen-containing groups and unique hierarchical porous structure endow the RHHPC with a high capacitance (278 F g−1 at 0.5 A g−1) and excellent rate capability (77.2% retention at 20 A g−1) in 6 mol L−1 KOH. Furthermore, the symmetric supercapacitor fabricated with RHHPC delivered a high energy density of 7.4 W h kg−1 at a power density of 6195 W kg−1, which reveals the promising application of RHHPC in high-performance supercapacitors.
1. Introduction
Supercapacitors have received considerable attention because of their high power density, fast recharging capability and long cycle life.1–3 Porous carbon materials are considered to be the most promising electrode materials for supercapacitors owing to their excellent physicochemical stability, high surface area, good electrical conductivity, and low cost.4,5 The ideal porous carbon materials should have both high capacitance and good rate capability for the supercapacitors to possess both high energy density and high power density.6 But conventional porous carbon usually consists of a single kind of pore, which makes it difficult to exhibit high capacitance and good rate capability, simultaneously.7,8 Recently, hierarchical porous carbons (HPCs), with 3D structures composed of macropores, mesopores and micropores are strongly recommended for the fabrication of advanced supercapacitors. Hierarchical porous carbons exhibit the advantages of macropores, mesopores and micropores through a synergistic effect, so they can afford both high capacitance and good rate capability.9–13 A template with a special structure usually is used to fabricate hierarchical porous carbons.12–15 However, the preparation of such templates is generally complicated, expensive, and time-consuming. This restricts their commercial viability. Recently, some novel strategies (for example, co-assembly and self-templating methods) for the synthesis of hierarchical porous carbon were proposed to simplify the preparation of the template or avoid using a template.16,17 Among, the self-templating method for preparing hierarchical porous carbon has been widely investigated using natural biomass materials, such as banana peel, watermelon, hemp fiber, bacterial fiber, fish scale, human hair and so on.18–23 Natural biomass materials exhibit a fine structure which cannot be achieved through artificial synthesis merely. These fine structure of the natural biomass can be transformed into hierarchical porous structure. Besides, the utilization of renewable biomass to produce HPCs is critical to the sustainable development and environmental protection. Although HPCs have been prepared from various biomasses by many researchers, the shortage of raw material is one of the biggest obstacles for their application. Considering the extensive applications of supercapacitors, the development of HPCs from low-cost and abundant biomass could be very rewarding and competitive.Rice husk (RH) is a kind of low-cost and abundant biomass. The annual world production of RH is approximately 140 million tons.24 RH possesses unique organized structures and composition (silica). The vascular bundles in RH are natural macroporous channels used to transport water and nutrients for the growth of rice.25 The silica with unique 3D nano-structure as a natural template skeleton is distributed in RH.26 Obviously, the self-construction (with both macroporous vascular bundles and 3D nano-silica) of RH is a natural template structure. If hierarchical porous carbons are prepared by using the self-template structure of RH, it will realize the “template-free” preparation of hierarchical porous carbons. More importantly, this strategy is simple, low-cost and sustainable for large scale industrial production. In recent years, RH has been used to prepare porous carbon via traditional physical and chemical activation methods.27 Unfortunately, the self-template structure of RH is not fully utilized during preparation process. Namely, the prepared porous carbon does not simultaneously exhibit high specific surface area and hierarchical porous structure. The porous carbon prepared by traditional physical activation exhibits low specific surface area.28,29 Although the specific surface area of porous carbon is improved via traditional chemical activation, the pore size of prepared porous carbon exhibits narrow micropore-dominated distribution,30–32 which restricted its application in the supercapacitors with high power performance. Therefore, it is necessary to prepare porous carbon with high specific area and hierarchical porous structure via a novel method in which the self-template structure of RH is transformed into hierarchical porous structure and high specific area is obtained.
In this work, rice husk-based hierarchical porous carbon (RHHPC) with high specific surface area was prepared based on the self-template structure of RH via carbonization, NaOH-leaching and KOH activation. The as-obtained RHHPC maintained the structure of RH with 3D porous surface, parallel porous channels and displayed a specific surface area as high as 2804 m2 g−1, and a high-level oxygen-containing groups (with an oxygen content of 9.74 at%). Benefiting from the high specific surface area, large amount of oxygen-containing groups and unique hierarchical porous structure, the RHHPC exhibited high capacitance and excellent rate performance, which endows RHHPC with great potential in for high-performance supercapacitors.
2. Experimental
2.1. Materials
RH was obtained from a rice mill nearby Changchun city. All other chemical reagents used in this study were of analytical grade from Beijing Chemicals Co., Ltd. Nitrogen was of chemical grade (purity of 99%). Deionized water was used throughout the experiment.2.2. Preparation of RHHPC
The main processes of preparing RHHPC are schematically illustrated in Fig. 1. The RH was heated to 500 °C and pyrolyzed for 1 h in a tubular furnace under the protection of nitrogen, then the carbon-silica composite was obtained. The carbon-silica composite was treated with NaOH-leaching, namely, the carbon-silica composite was refluxed with a solution of 10 wt% NaOH at 100 °C for 2 h to gain template carbon, then the template carbon was added to KOH solution to obtain the mixture with a KOH-to-template carbon ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by weight. Next, the mixture was dried at 110 °C to obtain the KOH-impregnated template carbon. The KOH-impregnated template carbon was heated up to 700 °C for 1 h in an electrical furnace. The activated mixtures were washed with deionized water until the filtrate became neutral. The sample was finally dried overnight at 100 °C.
1 by weight. Next, the mixture was dried at 110 °C to obtain the KOH-impregnated template carbon. The KOH-impregnated template carbon was heated up to 700 °C for 1 h in an electrical furnace. The activated mixtures were washed with deionized water until the filtrate became neutral. The sample was finally dried overnight at 100 °C.
2.3. Characterizations of RHHPC
The porous characteristics of the prepared materials were measured by N2 adsorption at 77 K using an ASAP 2010 Micrometrics instrument. The specific surface area was calculated on the basis of the Brunauer–Emmett–Teller (BET) model, and the pore size distribution (PSD) was calculated from desorption branch isotherms by the Density Functional Theory. The pore volume was estimated from the adsorbed amount at a relative pressure (P/P0) of about 0.97. The morphologies and structural properties of the prepared materials were characterized by field emission scanning electron microscopy (FESEM; HITACHI SU8020) and transmission electron microscopy (TEM, JEOL, JEM 1200EX). The structural properties were investigated by X-ray diffraction (XRD, Rigaku D/max 2550), Raman spectra (Bruker VERTEX 70). The X-ray photoelectron spectroscopy (XPS) analysis was performed on the Thermo VG ESCALAB250 surface analysis system.2.4. Electrochemical measurements
The electrochemical performances of the electrodes were measured in two-electrode setup. Each electrode was composed of 85 wt% of RHHPC, 10 wt% of acetylene black and 5 wt% of polytetrafluoroethylene. The electrochemical characteristics of samples were determined by galvanostatic charge/discharge (GCD), cyclic voltammetry (CV), and electrochemical impedance spectroscopy (EIS) in 6 M KOH. GCD was carried out on an NEWARE-BTS4008 test station at different current densities from 0.5 A g−1 to 20 A g−1, with the voltage window from 0 to 1 V. The PARSTAT 2273 electrochemical system was used for CV and EIS measurements. CV was conducted between 0 and 1 V at increasing sweep rates from 5 to 1000 mV s−1. EIS measurement was conducted at 0 V by sweeping the frequencies from 100 kHz to 0.1 Hz range with a voltage amplitude of 10 mV.3. Results and discussion
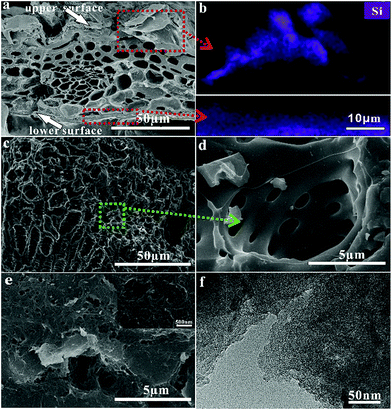
The RHHPC was prepared via carbonization, NaOH-leaching and KOH activation. Each process is a great need to obtain hierarchical porous structure from the self-template structure of RH.In the process of carbonization, the organic components of RH were pyrolyzed to form a stable porous structure. As shown in Fig. 2a, the self-construction of RH was perfectly preserved during carbonization process. Especially, the vascular bundles with channels from 2 to 10 μm were preserved very well as they appear in RH (see ESI, Fig. S1†). Meanwhile, a carbon-silica composite with nano-silica distributed in the upper and lower surface (the upper surface is the outer epidermis of rice husk and the lower surface is the inner epidermis of rice husk, Fig. S2†) was obtained in carbonization process (Fig. 2b). The obtained carbon-silica composite exhibited a low specific surface area of 19 m2 g−1 (Table S1†) owing to small amount of pore developed in carbonization process.
In order to utilize the self-template structure of RH, the carbon-silica composite was treated by NaOH-leaching. The nano-silica of carbon–silica composite was removed by NaOH, and the 3D nano-structure of silica was mapped in carbon matrix of the upper and lower surface. So, the template carbon with 3D structure on surface and inner parallel vascular bundles was obtained. The specific surface area of template carbon was increased to 247 m2 g−1 (Table S1†), which is much larger than that of carbon–silica composite. Although template carbon has unique structure, it suffers from low specific area leading to low capacitance.
The template carbon was activated by KOH for gaining hierarchical porous carbon with high specific surface area. The vascular bundles of RHHPC were still perfectly preserved after KOH activation (Fig. 2c). Besides, the relatively smaller macropores with pore diameter of 100–500 nm were clearly observed in the vascular walls (Fig. 2d). The surface of RHHPC exhibited 3D porous structure with different pore size (Fig. 2e). In addition, a large amount of micropores and mesopores were observed in RHHPC (Fig. 2f).
XRD and Raman spectroscopy were carried out to determine the structure of RHHPC. The broad peaks at approximately 22.5 and 43° in Fig. 3a, which can be attributed to typical reflections from the (002) and (100) planes of graphite, implies the amorphous structure of RHHPC.33 The two characteristic peaks at around 1330 and 1590 cm−1 exhibited in the Raman spectra (Fig. 3b) corresponded to the D-band (disordered carbon) and G-band (ordered graphite lattice), respectively.34,35 Besides, the ratio of the relative intensity of these two bands (ID/IG) is proportional to the number of defect sites in the graphite carbon.36,37 The lower the ratio is, the higher the graphitization is. It can be calculated that the ID/IG ratio of RHHPC is 1.26. The result confirmed that RHHPC has low graphitization degree. The low graphitization degree of RHHPC may be caused by the harsh KOH activation and doping of oxygen.
N2 adsorption–desorption isothermal analysis was performed to characterize the porous structures of RHHPC. RHHPC showed a combined I/IV type adsorption–desorption isothermal indicating that micro-, meso- and macropores coexist in RHHPC (Fig. 3c). A strong N2 adsorption occurs at low relative pressure of less than 0.1 due to the presence of micropores. The obvious hysteresis loop between the adsorption and desorption branches (at 0.4–0.8 P/P0) suggests the existence of mesopores. This result was in accordance with the PSD (insert of Fig. 3c). The PSD of RHHPC extended over the micropore and mesopore range, which is in good agreement with the observation results obtained by TEM. In addition, the slightly steep adsorption at the relative pressure of 0.8–1.0 demonstrated the presence of macropores. Calculated by the Brunauer–Emmett–Teller (BET) model, the specific surface area of RHHPC was as high as 2804 m2 g−1 with a pore volume up to 1.797 cm3 g−1. Such a high specific surface area can provide a sufficient electrode–electrolyte interface for the accumulation of ions. The surface chemistry of RHHPC was evaluated by XPS. The obvious C1s and O1s peaks were observed in the XPS spectra (Fig. 3d). The high-resolution O1s spectrum of RHHPC (Fig. 3e) can be deconvoluted into five individual component peaks corresponding to quinone type groups (530.4 eV), COOH/C(O)O (531.2 eV), C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (531.9 eV), –C–O (532.8 eV) and –OH (533.4 eV).38 The contents of individual oxygen-containing functional groups in RHHPC were summarized in Table S2.† The total surface oxygen content of RHHPC was 9.7 at%, showing the abundant oxygen-containing functional groups in the RHHPC. These oxygen-containing groups contribute to pseudo-Faradaic reactions and enable easy access of electrolyte species for the construction of double layer.39,40
O (531.9 eV), –C–O (532.8 eV) and –OH (533.4 eV).38 The contents of individual oxygen-containing functional groups in RHHPC were summarized in Table S2.† The total surface oxygen content of RHHPC was 9.7 at%, showing the abundant oxygen-containing functional groups in the RHHPC. These oxygen-containing groups contribute to pseudo-Faradaic reactions and enable easy access of electrolyte species for the construction of double layer.39,40
From the above results, it can be concluded that the RHHPC has the unique features such as hierarchical porous structure, high specific surface area and large amount of oxygen-containing groups. These unique features would endow RHHPC with excellent electrochemical performance.
Obviously, compared with porous carbon with dominant micropore derived from RH via traditional method, the obtained RHHPC exhibited hierarchical porous structure in our work. We believe that the development of hierarchical porous structure is related to the utilization of self-template structure of RH and the removal of silica. To clarify this, rice husk carbon (RHC) was prepared through traditional KOH activation process with the same annealing conditions as RHHPC (detailed in ESI†).
The obtained RHC exhibited irregular bulks rather than keeping the self-template structure of RH (Fig. S3†). The adsorption–desorption isotherm of RHC is approximately I type isotherm (Fig. S4†).41 It indicates that RHC possesses the high microporosity, which was different from RHHPC. This result is proved by pore size distribution of RHC (Fig. S5†). The reason is that the KOH reacts with silica on the upper and lower epidermis of RH and the new-born silicate generated from silica and KOH hinder the activation process.42 The process of forming macropores and mesopores structure from the 3D nano-structured carbon skeleton (due to the existence of nano-silica in the upper and lower surface of RH) by KOH etching was inhibited by defect activation. Meanwhile, this caused excessive etch and damage in the vascular bundles structure of carbon–silica composite. In consequence, the self-template structure of RH was not fully utilized in developing hierarchical porous structure via traditional KOH activation process.
Based on the above analysis, we proposed an explanation for how such hierarchical porous structure was formed. The formation process of hierarchical porous structure in RHHPC is shown in Fig. 4. In carbonization process, the carbon–silica composite with low specific surface area (Fig. 4b) was obtained due to the destruction of cellulose and hemicellulose in RH and the evaporation of volatile material in carbonization process.43 In NaOH-leaching process, template carbon (Fig. 4c) with 3D structure in surface (both upper and lower) and inner parallel vascular bundles was formed due to the removal of nano-silica in carbon–silica composite. The unique structure of template carbon was gradually transformed into hierarchical porous structure during KOH activation (detailed process as shown in Fig. 4c – e). At the beginning of KOH activation, active metallic potassium congregated in the porous channel of nano-structured carbon in surface and vascular bundles via capillary interaction (Fig. 4d).44 Along with the activation, the primary porous channels were enlarged forming macropores and large amounts of micropores and mesopores were formed in these macropores. When the activation reaction increased to some extent, the unconnected pores were interconnected. The hierarchical porous structure was obtained (Fig. 4e).
In order to verify the effectiveness of RHHPC as new electrode material for supercapacitors, CV, GCD, and EIS were measured in a 6 M KOH aqueous electrolyte.
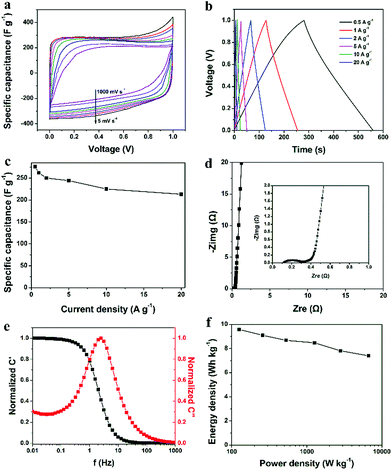
Fig. 5a presents the CV curves of RHHPC electrode at different scan rates from 5 to 1000 mV s−1. Notably, the quasi-rectangular shape of CV curves were well kept at a large range of scan rates and still exhibited a well symmetric shape even at 1000 mV s−1, indicating ideal double-layer capacitive properties. Fig. 5b shows the GCD curves for the RHHPC at different current densities. The GCD curves were nearly symmetric triangular shapes, indicating excellent capacitive behaviour. The gravimetric specific capacitance values at different current densities are shown in Fig. 5c. The corresponding specific capacitances of the electrode are calculated on the basis of the following equation:
 | (1) |
Fig. 5d shows the Nyquist plot based on a frequency response analysis of frequencies ranging from 0.01 Hz to 100 kHz. At low frequencies, the straight line tends to be perpendicular to the real axis, indicating the capacitive behavior.17 There is a semicircle on the impedance plot of RHHPC at high frequency (inset in Fig. 5d). The diameter of the semicircle along the real axis represents the charge-transfer resistance at the contact interface between the carbon and electrolyte in the pores of the electrode.8,48 A small semicircle indicated a low charge-transfer resistance. This is due to that the hierarchical porous structure of RHHPC and abundant oxygen-containing functional groups increase pore accessibility for the electrolyte. A small x-intercept (low equivalent series resistance (ESR)) was also observed for RHHPC in the high frequency region (inset in Fig. 5d). The ESR arises from the intrinsic electronic properties of the electrode matrix and electrolyte solution, mass transfer resistance of the ions in the matrix, and contact resistance between the current collector and the electrode.49,50 Such a low ESR of RHHPC is mainly due to the low contact resistance between the ion and material (where interconnected ion channels facilitate contact between the ion and material), and is also attributable to the good intrinsic electronic properties of the material.12 These results prove that the unique hierarchical porous structure can facilitate rapid electron and ion transport.51
The impedance frequency behavior was further investigated by the complex model of the capacitance (C(w)). The normalized C′(w) and C′′(w) as a function of frequency for the RHHPC electrodes are presented in Fig. 5e. It is obvious that the C′(w) is increased with the decrease of frequency, which can be attributed to the transformation from resistive behavior to capacitive behavior of RHHPC. In addition, the characteristic relaxation time constant defined as τ0 = 1/f0 (τ0, the minimum time to discharge all of the energy from a device with an efficiency of more than 50% (ref. 52)) is obtained from the frequency (f0) where C′′(w) is the maximum. The f0 is 2.51 Hz, corresponding to a τ0 of 398 ms. This result further demonstrates the fast accessibility of the RHHPC electrode to the electrolyte ions, the enhanced electrical conductivity and the excellent rate capability at high scan rates with high power density characteristics.
The energy density (E) and the power density (P) are important parameters to characterize the electrochemical performance of supercapacitor. Fig. 5f shows the Ragone plot of the symmetric supercapacitor based on the RHHPC electrodes. The energy density and power density can be calculated based on the following equations:
 | (2) |
 | (3) |
 | (4) |
As shown in Fig. 5f, with the increase of power density, energy density decreased slowly. When the power density increased from 125 to 6195 W kg−1, the energy density decreased from 9.6 to 7.4 W h kg−1. Based on the above results, it can be concluded that RHHPC electrode is capable of delivering high power without profound loss in energy, indicating a promising application in electrochemical capacitors. Furthermore, the cycling performance of RHHPC was investigated for as long as 6000 charge/discharge cycles. Fig. S7† displays the variations in discharge capacitance for the RHHPC electrode as a function of cycle number at a current density of 1 A g−1. As shown in Fig. S7,† the specific capacitance of RHHPC decreased slowly, and about 90.5% of the initial specific capacitance is retained after 6000 cycles. This demonstrates that the RHHPC has a relatively good long-term stability in the KOH electrolyte.
Such excellent electrochemical performance of RHHPC benefits from its high specific surface area, oxygen-containing groups and unique hierarchical structure. Firstly, high specific surface area affords sufficient electrode–electrolyte interface for the construction of electric double layer. Secondly, the oxygen-containing groups contribute to pseudo-Faradaic reactions and enable easy access of the electrolyte species for the construction of double layer; thirdly, the hierarchical porous nanostructure ensures the fast ion diffusion by shortening the diffusion pathways and using macropores frameworks as ion-buffering reservoirs, mesopores as ion-highways for fast ion transport and micropores for charge accommodation. These electrochemical performances will make the RHHPC material meet the need for high performance supercapacitors with high rate capability and power density.
4. Conclusions
Herein, we have developed a strategy for the preparation of hierarchical porous carbon from RH by carbonization, NaOH-leaching, and KOH activation, in which hierarchical structure was formed from the self-template structure of RH. The formation process of hierarchical structure in RHHC was proposed, where silica plays a crucial role. The template carbon with 3D structure in surface and inner parallel vascular bundles was obtained by the removal of silica. The unique structure of template carbon was gradually transformed into hierarchical porous structure during KOH activation. The as-obtained RHHPC maintained the structure of RH with 3D porous surface, parallel porous channels. Besides, RHHPC possessed high specific surface area (2804 m2 g−1). The RHHPC exhibited both a high specific capacitance (278 F g−1 at 0.5 A g−1) and excellent rate capability. The energy density of RHHPC based symmetric supercapacitor is as high as 7.4 W h kg−1 even at the power density of 6195 W kg−1. The RHHPC is a promising candidate material for high performance supercapacitors. Moreover, this strategy, using self-structure and component of biomass materials to prepare HPC, can provide new opportunities in developing carbon electrode materials for high-performance supercapacitors.Acknowledgements
The authors would like to acknowledge the support provided by key project in Jilin Province (20126010).References
- P. Hao, Z. H. Zhao, J. Tian, H. D. Li, Y. H. Sang, G. W. Yu, H. Q. Cai, H. Liu, C. P. Wong and A. Umar, Nanoscale, 2014, 6, 12120–12129 RSC.
- J. R. Miller and P. Simon, Science, 2008, 321, 651–652 CrossRef CAS PubMed.
- P. Simon and Y. Gogotsi, Nat. Mater., 2008, 7, 845–854 CrossRef CAS PubMed.
- Y. S. Tao, M. Endo and K. Kaneko, J. Am. Chem. Soc., 2009, 131, 904–905 CrossRef CAS PubMed.
- L. L. Zhang and X. Zhao, Chem. Soc. Rev., 2009, 38, 2520–2531 RSC.
- B. Xu, S. S. Hou, G. P. Cao, M. Chub and Y. S. Yang, RSC Adv., 2013, 3, 17500–17506 RSC.
- L. Qie, W. M. Chen, H. H. Xu, X. Q. Xiong, Y. Jiang, F. Zou, X. L. Hu, Y. Xin, Z. L. Zhang and Y. H. Huang, Energy Environ. Sci., 2013, 6, 2497–2504 Search PubMed.
- H. Zhong, F. Xu, Z. H Li, R. W. Fu and D. C. Wu, Nanoscale, 2013, 5, 4678–4682 RSC.
- F. Xu, R. J. Cai, Q. C. Zeng, C. Zou, D. C. Wu, F. Li, X. E. Lu, Y. R. Liang and R. W. Fu, J. Mater. Chem., 2011, 21, 1970–1976 RSC.
- D. W. Wang, F. Li, M. Liu, G. Q. Lu and H. M. Cheng, Angew. Chem., Int. Ed., 2008, 47, 373–376 CrossRef CAS PubMed.
- Y. Guo, Z. Q. Shi, M. M. Chen and C. Y. Wang, J. Power Sources, 2014, 252, 235–243 CrossRef CAS PubMed.
- Y. Y. Li, Z. S. Li and P. K. Shen, Adv. Mater., 2013, 25, 2474–2480 CrossRef CAS PubMed.
- T. C. Chou, C. H. Huang, R. A. Doong and C. C. Hu, J. Mater. Chem. A, 2013, 1, 2886–2895 CAS.
- Q. Wang, J. Yan, Y. B. Wang, T. Wei, M. L. Zhang, X. Y. Jing and Z. J. Fan, Carbon, 2014, 67, 119–127 CrossRef CAS PubMed.
- Y. Han, X. T. Dong, C. Zhang and S. X. Liu, J. Power Sources, 2012, 211, 92–96 CrossRef CAS PubMed.
- P. K. Tripathi, M. X. Liu, Y. H. Zhao, X. M. Ma, L. H. Gan, O. Noonanb and C. Z. Yu, J. Mater. Chem. A, 2014, 2, 8534–8544 CAS.
- M. J. Li, C. M. Liu, H. B. Cao, H. Zhao, Y. Zhang and Z. J. Fan, J. Mater. Chem. A, 2014, 2, 14844–14851 CAS.
- Y. K. Lv, L. H. Gan, M. X. Liu, W. Xiong, Z. J. Xu, D. Z. Zhu and D. S. Wright, J. Power Sources, 2012, 209, 152–157 CrossRef CAS PubMed.
- X.-L. Wu, T. Wen, H.-L. Guo, S. Yang, X. Wang and A.-W. Xu, ACS Nano, 2013, 7, 3589–3597 CrossRef CAS PubMed.
- H. Wang, Z. Xu, A. Kohandehghan, Z. Li, K. Cui, X. Tan, T. J. Stephenson, C. K. King'ondu, C. M. B. Holt, B. C. Olsen, J. K. Tak, D. Harfield, A. O. Anyia and D. Mitlin, ACS Nano, 2013, 7, 5131–5141 CrossRef CAS PubMed.
- L.-F. Chen, Z.-H. Huang, H.-W. Liang, W.-T. Yao, Z.-Y. Yu and S.-H. Yu, Energy Environ. Sci., 2013, 6, 3331–3338 CAS.
- W. X. Chen, H. Zhang, Y. Q. Huang and W. Q. Wang, J. Mater. Chem., 2010, 20, 4773–4775 RSC.
- W. J. Qian, F. X. Sun, Y. H. Xu, L. H. Qiu, C. H. Liu, S. D. Wang and F. Yan, Energy Environ. Sci., 2014, 7, 379–386 CAS.
- D. Kalderis, S. Bethanis, P. Paraskeva and E. Diamadopoulos, Bioresour. Technol., 2008, 99, 6809–6816 CrossRef CAS PubMed.
- B. D. Park, S. G. Wi, K. H. Lee, A. P. Singh, T. H. Yoon and Y. S. Kim, Biomass Bioenergy, 2003, 25, 319–327 CrossRef CAS.
- D. S. Jung, M.-H. Ryou, Y. J. Sung, S. B. Park and J. W. Choi, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 12229–12234 CrossRef CAS PubMed.
- Y. Chen, Y. C. Zhu, Z. C. Wang, Y. Li, L. L. Wang, L. L. Ding, X. Y. Gao, Y. J. Ma and Y. P. Guo, Adv. Colloid Interface Sci., 2011, 163, 39–52 CrossRef CAS PubMed.
- C. Deiana, D. Granados, R. Venturini, A. Amaya, M. Sergio and N. Tancredi, Ind. Eng. Chem. Res., 2008, 47, 4754–4757 CrossRef CAS.
- S. Kumagai, Y. Shimizu, Y. Toida and Y. Enda, Fuel, 2009, 88, 1975–1982 CrossRef CAS PubMed.
- Y. P. Guo, S. F. Yang, K. F. Yu, J. Z. Zhao, Z. C. Wang and H. D. Xu, Mater. Chem. Phys., 2002, 74, 320–323 CrossRef CAS.
- X. J. He, P. H. Ling, J. S. Qiu, M. X. Yu, X. Y. Zhang, C. Yu and M. D. Zheng, J. Power Sources, 2013, 240, 109–113 CrossRef CAS PubMed.
- L. J. Kennedy, J. J. Vijaya and G. Sekaran, Ind. Eng. Chem. Res., 2004, 43, 1832–1838 CrossRef CAS.
- W. X. Chen, H. Zhang, Y. Q. Huang and W. K. Wang, J. Mater. Chem., 2010, 20, 4773–4775 RSC.
- A. Jänes, H. Kurig and E. Lust, Carbon, 2007, 45, 1226–1233 CrossRef PubMed.
- F. Bonhomme, J. C. Lassègue and L. Servant, J. Electrochem. Soc., 2001, 148, 450–458 CrossRef PubMed.
- M. X. Liu, L. H. Gan, W. Xiong, Z. J. Xu, D. Z. Zhu and L. W. Chen, J. Mater. Chem. A, 2014, 2, 2555–2562 CAS.
- M. X. Liu, L. H. Gan, W. Xiong, F. Q. Zhao, X. Z. Fan, D. Z. Zhu, Z. J. Xu, Z. X. Hao and L. W. Chen, Energy Fuels, 2013, 27, 1168–1173 CrossRef CAS.
- R. Arrigo, M. Havecker, S. Wrabetz, R. Blume, M. Lerch, J. McGregor, E. P. J. Parrott, J. A. Zeitler, L. F. Gladden, A. Knop-Gericke, R. Schlogl and D. S. Su, J. Am. Chem. Soc., 2010, 132, 9616 CrossRef CAS PubMed.
- W. Li, F. Zhang, Y. Dou, Z. Wu, H. Liu, X. Qian, D. Gu, Y. Xia, B. Tu and D. Zhao, Adv. Energy Mater., 2011, 1, 382–386 CrossRef CAS.
- P. J. Hall, M. Mirzaeian, S. I. Fletcher, F. B. Sillars, A. J. R. Rennie, G. O. Shitta-Bey, G. Wilson, A. Cruden and R. Carter, Energy Environ. Sci., 2010, 3, 1238–1251 CAS.
- L. Qie, W. M. Chen, Z. H. Wang, Q. G. Shao, X. Li, L. X. Yuan, X. L. Hu, W. X. Zhang and Y. H. Huang, Adv. Mater., 2012, 24, 2047–2050 CrossRef PubMed.
- J. B. Zhang, L. J. Jin, J. Cheng and H. Q. Hu, Fuel, 2013, 109, 2–8 CrossRef CAS PubMed.
- A. Bharadwaj, Y. Wang, S. Sridhar and V. Arunachalam, Curr. Sci., 2004, 87, 981–986 Search PubMed.
- Y. Y. Lv, F. Zhang, Y. Q. Dou, Y. P. Zhai, J. X. Wang, H. J. Liu, Y. Y. Xia, B. Tu and D. Y. Zhao, J. Mater. Chem., 2012, 22, 93–99 RSC.
- Q. Wang, Q. Cao, X. Y. Wang, B. Jing, H. Kuang and L. Zhou, J. Power Sources, 2013, 225, 101–107 CrossRef CAS PubMed.
- Y. Gao, W. L. Zhang, Q. Y. Yue, B. Y. Gao, Y. Y. Sun, J. J. Kong and P. Zhao, J. Power Sources, 2014, 270, 403–410 CrossRef CAS PubMed.
- X. T. Hong, K. S. Hui, Z. Zeng, K. N. Hui, L. J. Zhang, M. Y. Mo and M. Li, Electrochim. Acta, 2014, 130, 464–469 CrossRef CAS PubMed.
- Y. S. Yun, C. B. Im, H. H. Par, I. Hwang, Y. Tak and H. J. Jin, J. Power Sources, 2013, 234, 285–291 CrossRef CAS PubMed.
- M. M. Shaijumon, F. S. Ou, L. Ci and P. M. Ajayan, Chem. Commun., 2008, 2373–2375 RSC.
- L. Zhang, F. Zhang, X. Yang, G. K. Long, Y. P. Wu, T. F. Zhang, K. Leng, Y. Huang, Y. F. Ma, A. Yu and Y. S. Chen, Sci. Rep., 2013, 3, 1408–1446 Search PubMed.
- B. Z. Fang, J. H. Kim, M. S. Kim, A. Bonakdarpour, A. Lam, D. P. Wilkinson and J. S. Yu, J. Mater. Chem., 2012, 22, 19031–19038 RSC.
- P. Banerjee, I. Perez, L. Henn-Lecordier, S. B. Lee and G. W. Rubloff, Nat. Nanotechnol., 2009, 4, 292–296 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed preparation process of RHC; pore structure of RHHPC, RHC, template carbon and carbon–silica composite; SEM image of RHC; SEM image and digital photograph of RH; nitrogen adsorption–desorption isotherms of RHC; summary of oxygen containing functional groups in RHHPC; pore size distribution of RHC and RHHPC; relationships between the specific capacitance values and current density for RHC and RHHPC; comparison of the electrochemical performances of RHHPC and other reported porous carbon materials; galvanostatic charge/discharge cycling stability of RHHPC. See DOI: 10.1039/c4ra15111a |
| This journal is © The Royal Society of Chemistry 2015 |