Synthesis of recyclable, chemically cross-linked, high toughness, high conductivity ion gels by sequential triblock copolymer self-assembly and disulfide bond cross-linking†
Chengsha Weia,
Mingming Chenb,
Dong Liub,
Weiming Zhoub,
Majid Khanb,
Xibo Wub,
Ningdong Huangb and
Liangbin Li*ab
aCAS Key Laboratory of Soft Matter Chemistry, Department of Polymer Science and Engineering, University of Science and Technology of China, China. E-mail: lbli@ustc.edu.cn; Fax: +86-551-5141078
bNational Synchrotron Radiation Lab and College of Nuclear Science and Technology, University of Science and Technology of China, No. 96, Jinzhai Road, Hefei, Anhui 230026, China
First published on 6th February 2015
Abstract
In this article, we report the synthesis of a disulfide bonded reversibly chemically cross-linked ion gel with high toughness and conductivity by sequential triblock copolymer self-assembly and the subsequent oxidation of thiol groups. Through reversible thiol-disulfide exchange, the ion gels had both high toughness of chemicals and recyclability of physical cross-linked ion gels. The triblock copolymer (SOS-SH) was prepared as follows: first, the RAFT copolymerization of styrene and 4-vinylbenzyl chloride (VBC) using CTA–PEO–CTA as a bi-functional macroRAFT agent was performed to obtain a triblock copolymer (SOS-Cl); then, the chloride group of SOS-Cl was replaced by an azido group to obtain SOS-N3; and finally, the click reaction of SOS-N3 with O-ethyl-S-prop-2-ynyl carbonodithioate and subsequent aminolysis were conducted to obtain SOS-SH. The disulfide bonded reversibly chemically cross-linked ion gel could be re-dissolved when mixed with a little amount of mild reducing agent (e.g., DTT) in CH2Cl2 with vigorous stirring, which reformed again after the removal of solvent and oxidation of thiol groups. The ion gels could undergo the reduction–oxidation cycle at least twice with a little loss of ionic conductivity and toughness (less than 25%), exhibiting good recyclability. Raman measurements were performed to confirm the existence and the key role of disulfide bond on the recyclability.
Introduction
Ion gels, polymeric networks swollen with a substantial amount of ionic liquid (IL), have attracted considerable interest because of their use in electrochemical devices, including actuators, super-capacitors, solid electrolytes, transistors, batteries, solar cells, gas separation membranes, and organic gate dielectrics, in recent years due to their unique physicochemical properties such as high ionic conductivity, thin-film forming ability, flexibility, transparency, non-volatility, non-flammability, and wide electrochemical window, as well as exceptional thermal and (electro)chemical stability.1–4 In addition, the various combinations of different ionic liquid and polymer, as well as the easy modification of polymer, impart flexibility for designing materials with desired properties. There are different routes to prepare ion gels, such as the in situ polymerization of monomer/cross-linker mixtures in ionic liquids,5 chemically reacting difunctional macromolecules with functional cross-linkers,6 and the swelling of elastomeric films.7 As an alternative way to develop ion gels, the self-assembly of triblock copolymers in ionic liquids has been proven to be particularly effective and versatile.8–10 Usually, the triblock copolymers are ABA types. The ionic liquid insoluble A blocks aggregate to form cross-links and the soluble B blocks/ionic liquid form a continuous phase. The properties of ion gels, such as gel modulus, minimum gel concentration and ionic conductivity, may be tuned over broad ranges by various methods, including the simple adjustment of the lengths of the IL-philic B and IL-phobic A blocks, the concentration of polymer and variation of the sequences. The ion gels produced by the self-assembly of ABA triblock copolymers were usually physically cross-linked gels with non-covalent network structure. These physical gels could be processed as liquids, then solidified in situ, and made reversible upon external stimulus such as light11 and temperature.12 However, just because of the reversible noncovalent cross-linking, the physical gels lacked toughness when subjected to large strains, which was due to the chain pull-out of the A blocks.10,13,14 To solve this issue, Yuanyan Gu et al. permanently chemically cross-linked the A block after the physical ion gels were formed from an ABA triblock copolymer. Because the chemical cross-linking was confined to the non-conducting A domains, the ionic transport in the conducting B + IL continuous phase was not affected.15 However, just because of the irreversible permanent chemical cross-linking, the ion gels could not be recycled any more.Dynamic covalent bonds are covalent bonds, which could break and reform reversibly under suitable conditions with minimal side reactions.16–19 They combine the reversibility of supramolecular non-covalent bond and the robustness of covalent bond, although with generally relatively slower kinetics of bond cleavage and formation.17 Dynamic covalent bonds have increasingly been exploited for constructing diverse functional polymers such as thermo-, chemo-, mechano-, and photo-responsive dynamic polymers.20–22 As one of the dynamic covalent bonds, disulfide bonds are well known to be reduced to thiols in the presence of various reducing agents. The resulting thiols can be reversibly oxidized to disulfide bonds.23 Due to this reversible nature and mild transformation conditions, the reversible exchange of thiol-disulfide bond has widely been used for the preparation of reversible polymer micelles and hydrogels.24–26 It is possible and meaningful to prepare reversible chemically cross-linked ion gels with high toughness and high conductivity as well as with recyclability through the disulfide bonds because of their capacity to reversibly break and reform.
Reversible addition–fragmentation chain transfer (RAFT) polymerization is one of the most important controlled radical polymerization methods and is widely used to synthesize various well-defined macromolecular structures (namely, block copolymers and star polymers) and functional polymers.27–29 Moreover, as one of the most common click reactions, Cu(I) catalyzed Huisgen 1,3-dipolar cycloaddition reaction of azides and alkynes has found wide applications, including the post-functionalization of synthetic polymers, due to the tolerance to numerous functional groups, high yields, requirement of moderate reaction conditions in multiple solvents, and high specificity.30–32 Combining RAFT polymerization with azide–alkyne click reaction has been a powerful tool to synthesize various sophisticated functional polymers with well-defined structures.33–35
In this article, we prepared a disulfide bonded reversibly chemically cross-linked ion gel with high toughness and conductivity by a sequential triblock copolymer self-assembly and subsequent oxidation of thiols to disulfide bonds. The ABA type triblock copolymer (SOS-SH) was synthesized by combining RAFT polymerization with azide–alkyne click reaction. The reversibly chemically cross-linked ion gel could be re-dissolved when mixed with a little amount of mild reducing agent (e.g., DTT) in CH2Cl2 with vigorous stirring, and it reformed again after the removal of solvent and oxidation of thiols. The cross-linked ion gels could undergo the reduction–oxidation cycle at least twice with a slight loss of ionic conductivity and toughness (less than 25%), exhibiting good recyclability.
Experimental
Chemical and materials
All the commercially available reagents were used as received unless otherwise noted. Poly(ethylene oxide) (PEO) (Mn = 35 kDa, Đ = 1.04) was purchased from Sigma-Aldrich and dehydrated by freeze-drying before use. 1-Ethyl-3-methylimidazolium bis-(trifluoromethyl) sulfonyl amide ([EMI][TFSA], ionic liquid), propargyl bromide (80% solution in toluene), dithiothreitol (DTT) and 4-vinylbenzyl chloride (VBC) were purchased from Aladdin-Reagent. All the other chemicals were purchased from Sinopharm Chemical Reagent Co., Ltd., China. Styrene (St) was purified by distillation under reduced pressure, and 4-vinylbenzyl chloride (VBC) was passed through activated alumina columns prior to use. Dichloromethane and triethylamine were dehydrated before use.Analyses
1H-NMR spectra were obtained on a Bruker Avance 400 (400 MHz) spectrometer using TMS as an internal reference. The molecular weight distributions (PDI, Mw/Mn) were determined by gel permeation chromatography (RI-GPC) with a PLgel Mixed-C column (Agilent Technologies). Agilent Technologies 1100 Series GPC Analysis System with Isocratic G1310A pump and RI-G1362A refractive index (RI) detector (set at 35 °C) was used. The eluent was tetrahydrofuran (THF) at a flow rate of 1.0 mL min−1. The polydispersity indexes (PDI, Mw/Mn) for the sample polymers were calibrated with standard polystyrene samples. The X-ray photoelectron spectra (XPS) were recorded on a Thermo-VG Scientific ESCALAB 250 XPS spectrometer with an MgKα X-ray source (1253.6 eV). The Raman spectrum was recorded using a RAMALOG 6 (SPEX, USA) with an argon ion laser at an excitation wavelength of 519 nm. Fourier transform infrared (FT-IR) spectra were recorded at room temperature using a TENSOR27 IR spectrometer (Bruker, Germany). All the spectra were recorded at a wavenumber resolution of 1 cm−1. Rheology and impedance measurements are described in detail below.Synthesis of the chain transfer agent (CTA), (S)-1-dodecyl-(S′)-(α,α′-dimethyl-α′′-acetic acid) trithiocarbonate
The chain transfer agent (CTA) was synthesized following a previously reported procedure with slight modifications.36 In a typical experiment, 1-dodecanethiol (8.08 g, 0.04 mol), acetone (19.24 g, 0.33 mol), and Aliquot 336 (tricaprylylmethylammonium chloride, 0.65 g, 1.6 mmol) were mixed in a three-necked round-bottomed flask cooled to 10 °C under a nitrogen atmosphere. Sodium hydroxide solution (50%) (3.34 g, 42 mmol) was added over 20 min. The reaction was stirred for an additional 15 min before carbon disulfide (3.04 g, 0.04 mol) in acetone (4.04 g, 0.07 mol) was added over 20 min, during which the colour turned red. After 10 min, chloroform (7.13 g, 0.06 mol) was added in one portion, followed by dropwise addition of 50% sodium hydroxide solution (16 g, 0.2 mol) over 30 min. The reaction was stirred overnight. 60 mL of ionized water was added, followed by 10 mL of concentrated HCl to acidify the aqueous solution. Then, most of the residual acetone was removed under reduced pressure. The solid was collected with a Buchner funnel and then stirred in 200 mL of 2-propanol. The undissolved solid was filtered-off and the 2-propanol solution was concentrated to dryness. Then, the resulting solid was re-dissolved in ethyl acetate and passed through a silica column with ethyl acetate as an eluent. After the solvent was completely removed under vacuum, the obtained crude product was recrystallized from hexane to afford a yellow crystalline solid (about 50% yield). 1H-NMR (in CDCl3) of this CTA is shown in Fig. 3A.Synthesis of O-ethyl-S-prop-2-ynyl carbonodithioate
O-Ethyl-S-prop-2-ynyl carbonodithioate was synthesized according to a previously reported procedure37 with slight modifications. In a typical experiment, potassium ethyl xanthogenate (5.00 g, 3 × 10−2 mol), 80% solution of propargyl bromide in toluene (5.10 g, 3.5 × 10−2 mol), and THF (50 mL) were placed in a 150 mL round-bottomed flask covered with aluminum foil. The reaction mixture was stirred overnight at room temperature. A white precipitate was formed (KBr). After filtration, the reaction mixture was diluted with 200 mL of deionized water. The product was extracted with diethyl ether (2 × 100 mL). The etherous layer was dried with anhydrous magnesium sulfate and concentrated. The product was purified using silica column chromatography (petroleum ether (30–60 °C): CH2Cl2 = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and freeze dried to obtain a pale-yellow oil (about 60% yield). 1H-NMR (in CDCl3): (δ = 1.44 ppm (t, 3H), δ = 2.23 ppm (t, 1H), δ = 3.87 ppm (d, 2H), δ = 4.67 ppm (q, 2H)).
1) and freeze dried to obtain a pale-yellow oil (about 60% yield). 1H-NMR (in CDCl3): (δ = 1.44 ppm (t, 3H), δ = 2.23 ppm (t, 1H), δ = 3.87 ppm (d, 2H), δ = 4.67 ppm (q, 2H)).
Polymer synthesis
 | ||
| Fig. 1 Synthesis procedure of bi-functional macroRAFT agent, CTA–PEO–CTA, and ABA type triblock copolymers, SOS-Cl, SOS-N3, SOS-triazole, and SOS-SH. | ||
CTA–PEO–CTA was then used as a bi-functional macroRAFT agent to copolymerize styrene and VBC (molar ratio 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) into the end-blocks. CTA–PEO–CTA (5.00 g, 0.143 mmol), purified styrene (5.50 g, 0.053 mol) and VBC (2.00 g, 0.013 mol) were added to a 25 mL tube with a stirring bar, degassed via three freeze–pump–thaw cycles, sealed under vacuum and heated in an oil bath at 60 °C for 30 minutes to obtain a homogeneous solution. The solution was then heated to 140 °C and polymerization was allowed to proceed for 42 min, followed by quenching with ice water. The reaction mixture was diluted in ca. 25 mL of CH2Cl2 and precipitated into n-hexane twice. The product (SOS-Cl) was then collected and freeze-dried under vacuum.
1) into the end-blocks. CTA–PEO–CTA (5.00 g, 0.143 mmol), purified styrene (5.50 g, 0.053 mol) and VBC (2.00 g, 0.013 mol) were added to a 25 mL tube with a stirring bar, degassed via three freeze–pump–thaw cycles, sealed under vacuum and heated in an oil bath at 60 °C for 30 minutes to obtain a homogeneous solution. The solution was then heated to 140 °C and polymerization was allowed to proceed for 42 min, followed by quenching with ice water. The reaction mixture was diluted in ca. 25 mL of CH2Cl2 and precipitated into n-hexane twice. The product (SOS-Cl) was then collected and freeze-dried under vacuum.
SOS-Cl (4.50 g) and NaN3 (0.80 g, 0.0123 mol) were then re-dissolved in N,N-dimethylformamide (ca. 200 mL) at 60 °C and the reaction was stopped after stirring for an additional 40 hours. Deionized water (ca. 5 mL) was added to quench the reaction. Most of the DMF was removed at about 120 °C under reduced pressure. The contents were re-dissolved in CH2Cl2 and washed with NaCl aqueous solution twice, followed by the addition of anhydrous magnesium sulfate to dry the organic phase. The product (SOS-N3) was obtained by precipitation in diethyl ether after most of the solvent was removed under vacuum at room temperature and freeze-dried under vacuum.
Ion gels preparation
Stock solutions to prepare ion gels were prepared by mixing weighed amounts of polymer (SOS-SH, 10 wt% of ion liquid) with an ionic liquid ([EMI][TFSA]) in CH2Cl2 (if the SOS-SH was not easily dissolved in CH2Cl2, a little amount of DTT was added (1.0 wt% of SOS-SH) and the mixture was subjected to ultrasonication and stirring was performed for 3 hours) and stocked in a desiccator. The ion gels were prepared using solvent casting. Weighed amounts of stock solution were placed in a desiccator for 48 hours to remove most of the solvent. The residual solvent was completely removed by freeze-drying for 1 hour. Completely cross-linked samples were obtained after allowing the gel to stand at 60 °C for an additional 24 h. Samples for extensional rheology were prepared following the same procedure for the less cross-linked samples as above, and then pressed into a 1 mm thick mould and annealed at ca. 60 °C for 1 hour (less cross-linked) or 24 hours (completely cross-linked). The samples were then carefully withdrawn and cut into pieces with the desired dimensions (10 mm long and 5 mm wide). To avoid moisture effects, all the ion gel samples were kept in a desiccator under vacuum.Reduction and reforming of cross-linking ion gels
The cross-linked ion gels, dithiothreitol (DTT) (5 wt% of SOS-SH), and CH2Cl2 were charged in a round-bottom flask and subjected to vigorous stirring at room temperature for 24 h. Subsequently, the re-cross-linked ion gel samples could be obtained following the same procedure described above. The second reduction and cross-linking were performed according to the same steps.Rheology
Dynamic shear rheological measurements were performed on a rotational rheometer (TAAR2000EX, TA Instruments, New Castle, DE) with a parallel plate geometry of 25 mm in diameter and a gap of ca. 1 mm in a nitrogen atmosphere. Dynamic frequency sweep measurements were carried out at 100 °C in the linear viscoelastic regime from the frequency of 100 to 0.1 rad s−1 with a strain of 3% and shear rate of 10 rad s−1. The less cross-linked gel sample used for the measurement was not subjected to the annealing of 1 hour (60 °C).Extensional rheological measurements (tensile tests) were performed on a homemade miniature tensile tester38 at room temperature. A schematic of the homemade miniature tensile tester used in this work is shown in Fig. 2. The sample was mounted between two clamps, which were driven through the rotation of the motor and gears. The force was measured through the sensor that connected the clamp and a stretching arm. Tests were conducted at room temperature (about 25 °C). The samples were stretched at the same rate (20 μm s−1), and the instrument measured the resulting extensional stress until the samples broke. The results of tensile tests are listed in Table S1 (ESI†).
 | ||
| Fig. 2 Schematic of the homemade miniature tensile tester: 1, 2, 3, 4, and 5 are motor, gears, sensor, clamps, and sample, respectively. | ||
Impedance analysis
Ionic conductivities were measured by AC impedance method according to the previous literature10 using a CHI660C electro-chemistry workstation (Shanghai CH Instruments Co., Ltd., China) with a homemade cell. Frequency sweeps were conducted from 1 to 105 Hz with an AC amplitude of 10 mV. The cell was composed of a Teflon spacer with an inner diameter of 0.7 cm and a thickness of 0.24 cm sandwiched between two stainless steel electrodes. Temperatures were controlled using an oven. The samples were thermally equilibrated for 5 min at each temperature prior to the measurements. Measurements were performed at a series of increasing temperatures from 30 to 100 °C. Ionic conductivity was determined from the high frequency plateau of the real part of the complex conductivity.Results and discussion
Syntheses and characterizations of chain transfer agent (CTA) and CTA–PEO–CTA, SOS-Cl, SOS-N3, SOS-triazole, SOS-SH
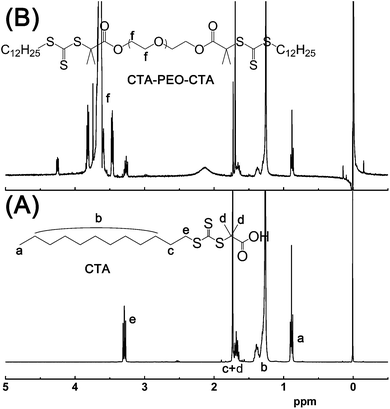
The syntheses of bi-functional macroRAFT agent, CTA–PEO–CTA, and ABA type triblock copolymers, SOS-Cl, SOS-N3, SOS-triazole, and SOS-SH, were carried out according to the route shown in Fig. 1.The bi-functional macroRAFT agent CTA–PEO–CTA was synthesized through the esterification of hydroxyl and carbonyl chloride. The successful synthesis of CTA–PEO–CTA was confirmed by the comparison of 1H-NMR of CTA (Fig. 3A) and CTA–PEO–CTA (Fig. 3B). For the 1H-NMR of CTA–PEO–CTA, the multiplet peak f was assigned to the methylene group (–O–CH2–) of PEO chain; moreover, the peaks of CTA (peaks at about 3.27, 1.71, 1.67, 1.26, and 0.88 ppm, compared to Fig. 3A) existed as well, indicating the successful attachment of CTA to the two ends of PEO chain.
 | ||
| Fig. 3 1H-NMR (in CDCl3) of (A) chain transfer agent (CTA) and (B) bi-functional macroRAFT agent, CTA–PEO–CTA. | ||
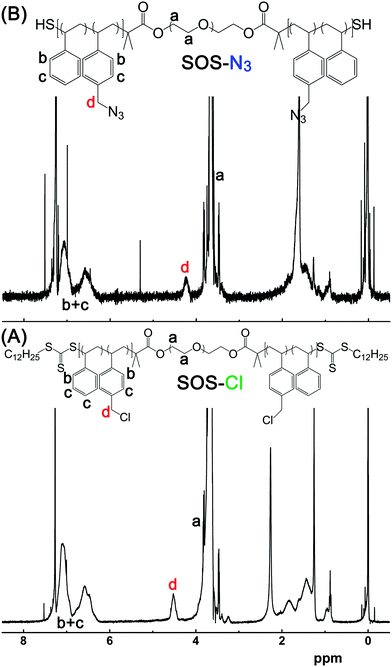
The triblock copolymer SOS-Cl was synthesized by the RAFT copolymerization of St and VBC using CTA–PEO–CTA as bi-functional macroRAFT agent. The successful synthesis of SOS-Cl was confirmed by 1H-NMR of SOS-Cl (Fig. 4A). Compared to the 1H-NMR of CTA–PEO–CTA (Fig. 3B), other than the peaks of PEO chain (about 3.64 ppm), additional broad peaks at about 4.516 ppm and 6.234–7.234 ppm also existed, which were assigned to the methylene group (–CH2–Cl) and phenyl group of poly (St-co-VBC)15 attached to the two ends of PEO chain, respectively.
The triblock copolymer SOS-N3 was synthesized by replacing the chloride group of SOS-Cl with an azide group using NaN3. The successful synthesis of SOS-N3 was confirmed by comparing 1H-NMR of SOS-Cl (Fig. 4A) and SOS-N3 (Fig. 4B). The peak of the methylene group (peak d, Fig. 4) shifted from 4.516 ppm (Fig. 4A) to 4.28 ppm (Fig. 4B), inferring the successful replacement of chloride group with azide group. The FT-IR spectrum of SOS-N3 also showed the characteristic absorption peak of the azide group (about 2100 cm−1, Fig. S1, ESI†), which was another strong supporting evidence.
The triblock copolymer SOS-triazole was synthesized by the click reaction of azide groups of SOS-N3 and alkynyl groups of O-ethyl-S-prop-2-ynyl carbonodithioate. The successful synthesis of SOS-triazole was confirmed by 1H-NMR and FT-IR spectra of SOS-triazole. As shown in the 1H-NMR of SOS-triazole (Fig. 5A), the peak of the methylene group (peak d) shifted from 4.28 ppm (Fig. 4B) to 5.36 ppm, along with the disappearance of the FT-IR characteristic absorption peak of the azide group of SOS-triazole (Fig. S1, ESI†), indicating the transformation of the adjacent azide group to another functional group. The 1H-NMR of the SOS-triazole also showed the characteristic peak of triazole group (peak f, 8.03 ppm) and some peaks were assigned to O-ethyl-S-prop-2-ynyl carbonodithioate (peaks g and h, 4.49 ppm and 4.63 ppm, respectively),37 indicating the transformation of the adjacent azide group to a triazole group, as well as the successful attachment of O-ethyl carbonodithioate group. All these observations strongly supported the successful synthesis of SOS-triazole.
The final ABA type triblock copolymer SOS-SH was synthesized by the aminolysis of SOS-triazole. The successful synthesis of SOS-SH was confirmed by comparing the 1H-NMR of SOS-triazole (Fig. 5A) and SOS-SH (Fig. 5B). As shown in Fig. 5B, the shift of peak g (the resulting peak g was covered by the giant peak a) and disappearance of peak h indicated the successful aminolysis of SOS-triazole. Because of the residual traces of copper salt, the resulting thiol groups were easily oxidised to disulfide bonds in air.39 Thus, the Raman spectrum of SOS-SH did not show the characteristic scattering peak of thiol (about 2600 cm−1, Fig. S4, ESI†), but it exhibited the peak of disulfide bond (about 460 cm−1 (Fig. 10 and S4†); this wavenumber was relatively lower than the general scattering peak of the disulfide bond and related discussion is given below in detail).
Molecular weights (Mn) and polydispersity index (PDI) of the ABA type triblock copolymer, SOS-Cl and SOS-N3
The molecular weights (Mn) of the triblock copolymers (SOS-Cl and SOS-N3) were calculated from 1H-NMR spectroscopy (Fig. S2, ESI†) by the integration of the methylene groups in PEO mid-block (peak a) and the hydrogen on phenyl rings (peak b + c) and the methylene group (–CH2–Cl or –CH2–N3, peak d) in the poly(St-co-VBC)-based end-blocks. The polydispersity indexes (PDI) of CTA–PEO–CTA, SOS-Cl and SOS-N3 were determined by gel permeation chromatography (GPC, Fig. S3, ESI†). The results are listed in Table 1.Preparation and characterization of disulfide bonded reversibly chemical cross-linking ion gel
The preparation of disulfide bonded reversibly chemically cross-linked ion gel was carried out according to the procedure described above, which is graphically shown in Fig. 6. The triblock copolymer, SOS-SH and the ionic liquid were first mixed in CH2Cl2. After the volatilization of the solvent, a less chemically cross-linked ion gel formed and total chemical cross-linking could be obtained after annealing for 24 hours (60 °C). The dynamic shear rheology, ionic conductivity, and tensile measurement results of less cross-linked and completely cross-linked ion gels are shown in Fig. 7, 8 and 9, respectively. The numbers 0, 1, and 2 in the brackets in Fig. 7, 8 and 9 indicate the times of reduction–oxidation cycle. | ||
| Fig. 6 Schematic preparation procedure of disulfide bonded cross-linked ion gel combining the self-assembly of ABA type triblock copolymer in ionic liquid and reversible chemical cross-linking. | ||
For the rheological measurement (Fig. 7, at 100 °C), both less cross-linked (black curve) and completely cross-linked (0) (red curve) ion gels displayed higher storage modulus (G′) than loss modulus in the measured temperature range, strongly demonstrating the formation of gels. After complete cross-linking, the gels showed a larger storage modulus than the less cross-linked ion gels. In terms of ionic conductivity (Fig. 8), the ionic conductivity of ion gel after complete cross-linking (0) (red points) was higher than the less cross-linked ion gel (black points) at all the measured temperature points, which maybe because of the shrinkage of non-conducting end-block domains. Remarkably, the ion gel after complete cross-linking (0) exhibited almost equal ion conductivity to the pure ionic liquid (namely, [EMI][TFSA]; magenta points), showing the excellent capability of ionic conduction. For the tensile measurement (Fig. 9, Table S1†), the completely cross-linked (0) (red curve) ion gels showed almost equal average ultimate elongation to the less cross-linked ion gels (black curve) (about 5.86, 5.9, respectively), but they exhibited higher average tensile strength (about 93.8 kPa and 75.0 kPa, respectively).
Recyclability investigation of the disulfide bonded reversibly chemical cross-linking ion gel
The recyclability of our ion gels could be evaluated by two factors: the allowed times of reduction–oxidation cycle and the performance loss during each time. Our disulfide bonded reversibly chemically cross-linked ion gels could be re-dissolved at least twice in CH2Cl2 after being reduced by DTT with vigorous stirring, and it could be re-oxidised to form chemically cross-linked gels after the solvent was removed, implying that the ion gels could be recycled at least twice. The performance loss of ion gels after the first and second reduction–oxidation cycle were assessed by rheology, ionic conductivity and tensile measurement (Fig. 7, 8 and 9, respectively) and the performance of the ion gel after cross-linking (0) was used as the reference.As shown in Fig. 7, after the first and second cycles, the ion gels still displayed gel states (G′ > G′′) even at 100 °C in the measured frequency range. The storage modulus (G′) of the ion gel after the second cycle (the dark cyan curve) was a little larger than the one after the first cycle (the blue curve) and both of them were also a little larger than the one after cross-linking (0) (red curves). For the tensile measurement (Fig. 9, Table S1†), the average tensile strength decreased a little to 86.5 kPa (blue curve) from 93.8 kPa (red curve) and the average ultimate elongation also decreased by 13% (from 5.9 to 5.1) after the first cycle compared to the ones after cross-linking (0). However, after the second cycle (dark cyan curve), the tensile strength increased by 4% (from 93.8 to 97.5 kPa) and the ultimate elongation decreased by 15% (from 5.9 to 5). This showed that the strength (tensile strength and storage modulus) increased (less than 5%) after the reduction–oxidation cycle, but the toughness (ultimate elongation) decreased (less than 15%). In terms of the ionic conductivity (Fig. 8), after the first (blue points) and second cycle (dark cyan points), the ionic conductivity of the ion gels was maintained almost identical to the one after cross-linking (0) at higher temperatures (from 50 °C to 100 °C), but it slightly decreased (less than 25%) at lower temperatures (from 25 °C to 40 °C).
All these observations showed that after two reduction–oxidation cycles, the performance of ion gels did not undergo considerable loss (less than 25%), and the strength even increased by about 5%, inferring the good recyclability of this ion gel.
Raman spectra characterization of the disulfide bond in the ion gels
Although the fact that the ion gels could not be re-dissolved in CH2Cl2 after cross-linking and could be re-dissolved in CH2Cl2 after the mild reducing agent DTT was added strongly demonstrated that cross-linking was caused by the formation of disulfide bond, more clear evidence was needed. Raman spectroscopy is an important method for determining the presence of disulfide bonds. To confirm the existing and key role of the disulfide bond, the Raman spectra of most of the intermediate products were recorded and the result is shown in Fig. 10. The characteristic scattering peak of S–S stretching vibration in the Raman spectrum is located in the region of 430–550 cm−1.40 Thus, we focused on this region. | ||
| Fig. 10 Raman spectra of SOS-N3, SOS-triazole, SOS-SH, ion gel (0), ion gel (1), ion gel (2). The numbers (0, 1, and 2) in the brackets indicate the times of reduction–oxidation cycle. | ||
As shown in Fig. 10, the Raman spectra of SOS-N3 and SOS-triazole did not display obvious peaks in this region, fitting with no disulfide bond structure in their structural formula. The obvious peak in this region first appeared in the spectrum of SOS-SH, which was located at about 460 cm−1. Because of the presence of residual traces of copper salt, the resultant thiols were easily oxidised to disulfide bonds in air and the spectrum (Fig. S4, ESI†) did not show the characteristic scattering peak of S–H stretching vibration (at about 2600 cm−1). Although this value was relatively lower than the general scattering peak value of disulfide bond (about 500 cm−1), we still consider it to be the scattering peak of disulfide bond because this value was located in the desired region (430–550 cm−1), and the XPS of S2p of SOS-SH (Fig. S5, ESI†) displayed the peaks assigned to –S–S– or S–H.41 The spectrum of ion gel after cross-linking (ion gel (0)) also exhibited this peak at the same position. The spectra of ion gels after the first and second reduction–oxidation cycles (ion gel (1) and ion gel (2)) also displayed the peaks of disulfide bond, but shifted to 495 cm−1 (the red circles in Fig. 10). Although the peaks were not very obvious, we could consider it to be the scattering peak of disulfide bond when the other peaks are compared with the ones of SOS-SH and ion gel (0), especially the peaks in the black ellipses in Fig. 10. The shift of the peak may be because of the change of the disulfide bond dihedral angles42 when its structure changed from that shown in Fig. S6A (ESI†) to that shown in Fig. S6B (ESI†) after the addition of DTT. All these observations give the direct evidence of the existence of the disulfide bond, strongly confirming the key role it plays in the cross-linking and recyclability of the ion gels.
Conclusions
In conclusion, we have prepared a disulfide bonded reversibly chemically cross-linked ion gel by sequential triblock copolymer self-assembly and subsequent oxidation of thiols. The triblock copolymer SOS-SH was synthesized by combining RAFT polymerization and click reaction. 1H-NMR, FT-IR, Raman spectra, XPS, and GPC strongly confirmed the successful synthesis and relative low DPI of SOS-SH. The results of dynamic shear rheological measurements confirmed the formation of gels (G′ > G′′). The first cross-linking ion gels showed high tensile strength (about 0.1 MPa), high ultimate elongation (about 6), and high ionic conductivity (about 7 mS cm−1). After two reduction–oxidation cycles, the performance loss (toughness and ionic conductivity) of the ion gels was little (less than 25%) and the strength even increased by about 5%, inferring the excellent recyclability of this ion gel. The Raman spectra gave the direct evidence of the existence of the disulfide bond, confirming the key role it played on the cross-linking and recyclability of the ion gels.Acknowledgements
This work is supported by the National Natural Science Foundation of China (51325301, U1232129, 11275205 and 11204285), 973 program of MOST (2010CB 934504), special financial grant from the China postdoctoral science foundation (2012M521255), and the Fundamental Research Funds for the Central Universities (WK2310000025).Notes and references
- S. Imaizumi, H. Kokubo and M. Watanabe, Macromolecules, 2012, 45, 401–409 CrossRef CAS.
- S. Zhang, K. H. Lee, J. Sun, C. D. Frisbie and T. P. Lodge, Macromolecules, 2011, 44, 8981–8989 CrossRef CAS.
- Y.-S. Ye, J. Rick and B.-J. Hwang, J. Mater. Chem. A, 2013, 1, 2719–2743 CAS.
- A. S. Shaplov, P. S. Vlasov, M. Armand, E. I. Lozinskaya, D. O. Ponkratov, I. A. Malyshkina, F. Vidal, O. V. Okatova, G. M. Pavlov, C. Wandrey, I. A. Godovikov and Y. S. Vygodskii, Polym. Chem., 2011, 2, 2609–2618 RSC.
- M. A. B. H. Susan, T. Kaneko, A. Noda and M. Watanabe, J. Am. Chem. Soc., 2005, 127, 4976–4983 CrossRef CAS PubMed.
- M. A. Klingshirn, S. K. Spear, R. Subramanian, J. D. Holbrey, J. G. Huddleston and R. D. Rogers, Chem. Mater., 2004, 16, 3091–3097 CrossRef CAS.
- J. C. Jansen, K. Friess, G. Clarizia, J. Schauer and P. Izaák, Macromolecules, 2010, 44, 39–45 CrossRef.
- T. P. Lodge, Science, 2008, 321, 50–51 CrossRef CAS PubMed.
- J. Lee, M. J. Panzer, Y. He, T. P. Lodge and C. D. Frisbie, J. Am. Chem. Soc., 2007, 129, 4532–4533 CrossRef CAS PubMed.
- Y. He, P. G. Boswell, P. Bühlmann and T. P. Lodge, J. Phys. Chem. B, 2006, 111, 4645–4652 CrossRef PubMed.
- T. Ueki, Y. Nakamura, A. Yamaguchi, K. Niitsuma, T. P. Lodge and M. Watanabe, Macromolecules, 2011, 44, 6908–6914 CrossRef CAS.
- Y. He and T. P. Lodge, Chem. Commun., 2007, 2732–2734 RSC.
- M. E. Seitz, W. R. Burghardt, K. T. Faber and K. R. Shull, Macromolecules, 2007, 40, 1218–1226 CrossRef CAS.
- G. Tae, J. A. Kornfield, J. A. Hubbell and J. Lal, Macromolecules, 2002, 35, 4448–4457 CrossRef CAS.
- Y. Gu, S. Zhang, L. Martinetti, K. H. Lee, L. D. McIntosh, C. D. Frisbie and T. P. Lodge, J. Am. Chem. Soc., 2013, 135, 9652–9655 CrossRef CAS PubMed.
- J.-F. Xu, Y.-Z. Chen, L.-Z. Wu, C.-H. Tung and Q.-Z. Yang, Org. Lett., 2013, 15, 6148–6151 CrossRef CAS PubMed.
- Y. Jin, C. Yu, R. J. Denman and W. Zhang, Chem. Soc. Rev., 2013, 42, 6634–6654 RSC.
- A. W. Jackson and D. A. Fulton, Polym. Chem., 2013, 4, 31 RSC.
- M. Pepels, I. Filot, B. Klumperman and H. Goossens, Polym. Chem., 2013, 4, 4955–4965 RSC.
- Y. Amamoto, Y. Higaki, Y. Matsuda, H. Otsuka and A. Takahara, J. Am. Chem. Soc., 2007, 129, 13298–13304 CrossRef CAS PubMed.
- J. F. Folmer-Andersen and J.-M. Lehn, J. Am. Chem. Soc., 2011, 133, 10966–10973 CrossRef CAS PubMed.
- M. E. Belowich and J. F. Stoddart, Chem. Soc. Rev., 2012, 41, 2003–2024 RSC.
- L. Sun, J. Liu and H. Zhao, Polym. Chem., 2014, 5, 6584–6592 RSC.
- C. Ferris, M. Violante de Paz, A. Aguilar-de-Leyva, I. Caraballo and J. A. Galbis, Polym. Chem., 2014, 5, 2370–2381 RSC.
- T. C. Lai, H. Cho and G. S. Kwon, Polym. Chem., 2014, 5, 1650–1661 RSC.
- B. Nottelet, G. Tambutet, Y. Bakkour and J. Coudane, Polym. Chem., 2012, 3, 2956–2963 RSC.
- J. J. Haven, C. Guerrero-Sanchez, D. J. Keddie, G. Moad, S. H. Thang and U. S. Schubert, Polym. Chem., 2014, 5, 5236–5246 RSC.
- C. Wei, M. Chen, J. Tao, X. Wu, M. Khan, D. Liu, N. Huang and L. Li, Polym. Chem., 2014, 5, 7034–7041 RSC.
- G. Moad, M. Chen, M. Haussler, A. Postma, E. Rizzardo and S. H. Thang, Polym. Chem., 2011, 2, 492–519 RSC.
- R. Ranjan and W. J. Brittain, Macromol. Rapid Commun., 2007, 28, 2084–2089 CrossRef CAS.
- S. V. Orski, G. R. Sheppard, S. Arumugam, R. M. Arnold, V. V. Popik and J. Locklin, Langmuir, 2012, 28, 14693–14702 CrossRef CAS PubMed.
- H. Durmaz, A. Sanyal, G. Hizal and U. Tunca, Polym. Chem., 2012, 3, 825–835 RSC.
- J. Jin, J. Tian, X. Lian, P. Sun and H. Zhao, Soft Matter, 2011, 7, 11194 RSC.
- M. Li, P. De, S. R. Gondi and B. S. Sumerlin, Macromol. Rapid Commun., 2008, 29, 1172–1176 CrossRef CAS.
- Y. Huang, T. Hou, X. Cao, S. Perrier and Y. Zhao, Polym. Chem., 2010, 1, 1615–1623 RSC.
- J. T. Lai, D. Filla and R. Shea, Macromolecules, 2002, 35, 6754–6756 CrossRef CAS.
- N. Akeroyd, R. Pfukwa and B. Klumperman, Macromolecules, 2009, 42, 3014–3018 CrossRef CAS.
- W. Chen, X. Li, H. Li, F. Su, W. Zhou and L. Li, J. Mater. Sci., 2013, 48, 4925–4933 CrossRef CAS.
- A. Hiniker, J. F. Collet and J. C. Bardwell, J. Biol. Chem., 2005, 280, 33785–33791 CrossRef CAS PubMed.
- V. Lim, K. Peh and S. Sahudin, Int. J. Mol. Sci., 2013, 14, 24670–24691 CrossRef PubMed.
- D. G. Castner, K. Hinds and D. W. Grainger, Langmuir, 1996, 12, 5083–5086 CrossRef CAS.
- H. E. Van Wart, A. Lewis, H. A. Scheraga and F. D. Saeva, Proc. Natl. Acad. Sci. U. S. A., 1973, 70, 2619–2623 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: FT-IR spectra of SOS-N3 and SOS-triazole; integration of 1H-NMR spectra of SOS-Cl and SOS-N3; GPC traces of CTA–PEO–CTA, SOS-Cl and SOS-N3; Raman spectroscopy of SOS-SH; XPS of S2p of SOS-SH and ion gel (0); disulfide bond structure of SOS-SH, ion gel (0), ion gel (1) and ion gel (2); tensile test results. See DOI: 10.1039/c4ra15095c |
| This journal is © The Royal Society of Chemistry 2015 |