Cerium functionalized PVA–chitosan composite nanofibers for effective remediation of ultra-low concentrations of Hg(ii) in water†
Abstract
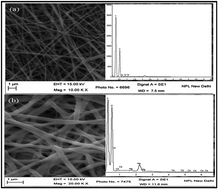
Mercury contaminated drinking water significantly affects the central nervous system, kidneys and other organs in humans even at very low concentration. Higher concentration of mercury are reported to be effectively removed by adsorption and precipitation techniques. Reverse osmosis (RO) is a better known technique used for the removal of low concentration of Hg (<200 ppb). However, its limitations include low flux, high water rejection, high capital cost, in addition to being power dependent. The present study reports the fabrication of low cost, biodegradable, electrospun cerium functionalized PVA–chitosan (Ce–PVA–CHT) composite nanofibers for the effective removal of low concentrations of Hg(II) present in water. It adsorbs Hg(II) and purifies water up to safe potable limits as prescribed by WHO/US-EPA. The adsorption of Hg(II) over the surface of Ce–PVA–CHT is confirmed by SEM/EDAX, FTIR, XRD and XPS techniques. The adsorption studies are reported by varying parameters, viz. time, pH, adsorbent dose and varying contents of Ce in PVA–CHT nanofibers. Traceability is established by using SCP Science-U.K. made certified reference standard for the calibration of AAS-HG used for the determination of Hg(II). The kinetic data shows fast and efficient removal of Hg(II) and indicates to follow pseudo second order kinetics. The adsorption data is best fitted to the Langmuir isotherm and indicates monolayer adsorption of Hg(II).

 Please wait while we load your content...
Please wait while we load your content...