Synergistic catalytic effect of the ZnBr2–HCl system for levulinic acid production using microwave irradiation†
Abstract
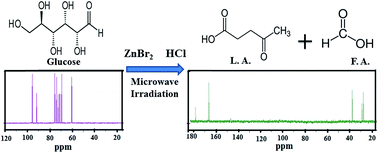
A catalytic process for the selective conversion of carbohydrates to levulinic acid is developed. A synergy in the catalytic action is observed when a combination of ZnBr2 and HCl was used as the catalyst which is attributed to the in situ generation of HBr. Carbohydrates, namely, glucose, molasses and sucrose, were employed as feedstock for levulinic acid production. Microwave irradiation of glucose either in the presence of HCl alone or both HCl and ZnBr2 as catalysts yielded the formation of levulinic acid. But the conversion of glucose to levulinic acid was much faster (only 6 min) when both HCl and ZnBr2 were employed together. The effect of the reaction parameters like, the time of irradiation, % power, and amount of substrate and catalyst on the yield of levulinic acid were studied. The reaction products in each case were analysed using 1H and 13C NMR. The yield of levulinic acid was estimated using HPLC. The maximum yield of levulinic acid obtained from glucose was 53 wt%.

 Please wait while we load your content...
Please wait while we load your content...