Effect of overexpression of endogenous and exogenous Streptomyces antibiotic regulatory proteins on tacrolimus (FK506) production in Streptomyces sp. KCCM11116P†
Chao Chena,
Xinqing Zhao*ab,
Liangyu Chena,
Yingyu Jinc,
Zongbao K. Zhaod and
Joo-Won Suhc
aSchool of Life Science and Biotechnology, Dalian University of Technology, Dalian 116024, China. E-mail: xqzhao@sjtu.edu.cn; Fax: +86-21-34208028; Tel: +86-21-34206673
bSchool of Life Science and Biotechnology, Shanghai Jiaotong University, Shanghai 200240, China
cDivision of Bioscience and Bioinformatics, Myongji University, Yongin 449-728, South Korea
dDepartment of Biotechnology, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian 116023, China
First published on 27th January 2015
Abstract
With the development of synthetic biology and systems biology, overproduction of biomolecules can be achieved by manipulation of regulatory proteins from various sources. FK506 (tacrolimus) is a 23-membered macrolide antibiotic and an important immunosuppressant. Overproduction of FK506 has been achieved by the manipulation of regulatory genes that are located inside the biosynthetic gene cluster, however, little is known on the effect of other regulators on FK506 production. In this study, the effects of three Streptomyces antibiotic regulatory proteins (SARPs) on FK506 production were investigated. These SARPs include bulZ and bulY that are cloned from a FK506 producer Streptomyces sp. KCCM11116P, and one novel SARP family regulatory protein Sx5140 that was obtained from a marine streptomycete S. xinghaiensis. The production titer of the engineered strains exhibited a higher production level compared to that in the control strain (227.99 mg L−1), and overexpression of bulZ resulted in the highest FK506 titer (365.59 mg L−1), which is 1.6-fold of that of the control. Real-time PCR analysis showed that overexpression of bulZ and bulY resulted in increased transcription of tsuR1 which is the gamma-butyrolactone receptor. Variation of transcription levels of fkbN, the positive regulator of FK506 biosynthesis, as well as fkbG, fkbH, fkbI, tcsA, tcsB, tcsD and fkbQ genes that are involved in the FK506 biosynthesis were observed in the SARP overexpression strains compared to those in the control strain. Our results demonstrate the promising potential to utilize alternative regulatory proteins including both endogenous and exogenous ones to enhance the production of useful compounds in microbial strains.
1 Introduction
FK506 (tacrolimus) is a 23-membered macrolide antibiotic which was first isolated from Streptomyces tsukubaensis.1 FK506 is well known as an immunosuppressant that is used in organ transplantation, and is also applied in the treatment of autoimmune diseases.2 Compared with cyclosporine, FK506 is more efficient and exerts fewer negative effects.3 The clinical applications of FK506, in addition to its economical profit (up to % 1635 million in 2013) have stimulated extensive studies to improve its bioproduction.4The biosynthesis of FK506 by various microbial producers has been explored in recent years.4 Three regulators (FkbN, FkbT and FkbR) are known to be located in FK506 biosynthetic gene cluster,5 and the effects of these regulators were evaluated in different FK506-producing strains. Among these three regulators, FkbN was reported to act as a positive regulator for FK506 production6,7 and was also applied to enhance FK506 production in a heterologous host.8 In addition, other genetic approaches have been also attempted to improve FK506 production. In Streptomyces sp. KCCM11116P, overexpression of the putative extracytoplasmic-function sigma factor FujE enhanced FK506 production.9 FK506 production was also stimulated by the combination of random mutagenesis and metabolite engineering of propionyl-CoA carboxylase pathway genes to increase precursor supply.10
Production of secondary metabolites in Streptomyces is regulated by a complex network of regulatory genes.11 Genes involved in the biosynthesis of secondary metabolites are generally clustered, and are co-regulated by both pathway-specific transcriptional regulators and global transcriptional regulators.11–14 Among several categories of regulatory genes involved in secondary metabolite production in streptomycetes, the Streptomyces antibiotic regulatory proteins (SARPs) are characterized by a unique OmpR-type wringed helix-turn-helix motif towards their N-terminal and a bacterial transcriptional activator domain (BTAD) at the C terminus.11,15 Most SARPs reported so far are pathway-specific regulatory proteins that can regulate antibiotic production. ActII-ORF4 and RedD in S. coelicolor A3 (2) are two well-known pathway-specific regulators of SARP family, which positively control the production of two pigments, namely actinorhodin (Act) and undecylprodigiosin (Red), respectively.16,17 DnrI is a SARP family regulator found in S. peucetius, which positively controls the biosynthesis of daunorubicin,18,19 and TylS is a SARP regulator reported to regulate production of tylosin in S. fradiae.20
In our previous study, a marine-derived streptomycete species S. xinghaiensis was characterized as a novel species21 and a large variety of putative regulatory proteins were identified in its genome.22 We selected one SARP encoding gene Sx5140 from S. xinghaiensis to study its effect on FK506 production. Sx5140 shows low similarity with other known sequences, and it locates closely to a putative antibiotic biosynthetic gene cluster. Interestingly, during our studies, partial genomic sequences of FK506 producer were unveiled,23,24 and two SARPs encoding bulZ and bulY25 showing high similarity with that of Sx5140 were identified. In this study, we reported the positive effects of overexpression of these three SARPs on FK506 production, and the underlying mechanisms were also explored by quantification of the transcription levels of related key genes.
2 Materials and methods
2.1 Strains, media and growth conditions
Streptomyces sp. KCCM11116P and its derivative strains were grown at 28 °C on ISP4 agar medium26 for spore collection. Spores were inoculated in the seed culture medium (glucose 1.0%, soluble starch 1.0%, yeast extract 1%, corn steep powder 0.5%, CaCO3 0.1%, pH 6.6) and cultivated at 28 °C, 200 rpm for 3 days, and 100 mL production medium (glucose 1.0%, dextrin 10%, dried yeast 1%, corn steep powder 0.5%, K2HPO4 2%, CaCO3 0.1%, pH 6.8) in 500 mL flask was used to inoculate each strain for FK506 production under the same condition. When necessary, 50 μg mL−1 thiostrepton was added in the culture medium. Protoplast transformants were regenerated on R2YE medium. Escherichia coli DH5α and E. coli ET12567 were grown at 37 °C in Luria–Bertani medium (LB), and ampicillin (100 μg mL−1) was added for selection of transformants. E. coli ET12567 was used to propagate non-methylated DNA. Strains used in this study were listed in Table S1.†2.2 DNA manipulations and sequences analysis
Routine DNA manipulations were carried out by standard methods.27 Protoplast preparation and transformation of Streptomyces species were performed as described by Kieser et al.28 Plasmids used in this study were listed in Table S1.†Analysis of the sequences of BulZ, BulY and Sx5140 with other SARP family members was performed by DNAMAN (Version 6).
2.3 Gene cloning and construction of overexpression plasmids
Genes of bulZ and bulY were cloned by PCR amplification using the genomic DNA of Streptomyces sp. KCCM11116P as a template, and gene Sx5140 was amplified from S. xinghaiensis. PCR product was purified and ligated into pMD-19T, then transformed into E. coli DH5α. Clones with ampicillin-resistant phenotype were chosen to extract plasmids which were subjected to sequencing analysis to identify the inserts with the correct bulZ, bulY and Sx5140 sequences, respectively. Subsequently, the confirmed pMD-19T-bulZ, pMD-19T-bulY and pMD-19T-Sx5140 were double digested to obtain inserts for construction of expression plasmids.Plasmid pWHM3 was used to construct expression plasmids. Plasmids were extracted from positive clones of E. coli DH5α with ampicillin-resistance, and the correct sequences in the constructed plasmids were verified by enzyme digestion and subsequent sequencing. Primers and restriction enzyme sites used in this study were listed in Table S2.†
2.4 Overexpression of SARPs encoding genes in Streptomyces sp. KCCM11116P
Plasmids of pWHM3, pWHM3-Sx5140, pWHM3-bulZ and pWHM3-bulY were introduced into Streptomyces sp. KCCM11116P by protoplast transformation, respectively. Four plasmids were demethylated using E. coli ET12567 before the transformation. Protoplast preparation and transformation were performed according to Kieser et al.28 Plasmids were prepared from the mycelia of the selected clones with thiostrepton-resistance, which were then transformed into E. coli DH5α to isolate plasmids for confirmation by enzyme digestion and sequencing.2.5 Fermentation of the recombinant strains for FK506 production
Spores of Streptomyces sp. CC01, Streptomyces sp. CC02, Streptomyces sp. CC03 and Streptomyces sp. C5140 were obtained on ISP4 agar cultured for 5 days at 28 °C. The seed culture was inoculated in seed medium for 3 days, and after adjusting the OD600 values of each seed culture to the same value (OD600 0.8), 10% (v/v) seed culture was inoculated into 100 mL main medium per 500 mL flask, followed by incubation at 200 rpm, 28 °C, and the samples were collected at 24 h intervals, starting at 48 h after inoculation.2.6 Analysis of FK506 production
Culture samples (2 mL) were centrifuged at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min, the supernatant was extracted by ethyl acetate (2 mL), whereas the mycelia was extracted by methanol (2 mL), after which the samples were evaporated to dryness under reduced pressure, and re-dissolved in 200 μL methanol.
000 rpm for 10 min, the supernatant was extracted by ethyl acetate (2 mL), whereas the mycelia was extracted by methanol (2 mL), after which the samples were evaporated to dryness under reduced pressure, and re-dissolved in 200 μL methanol.
The production levels of FK506 were determined by high-performance liquid chromatography (HPLC) as described previously by Mo et al.,29 and FK506 purchased from Sigma-Aldrich was used as a standard. The fermentation experiments were performed in triplicate, and samples were collected from three independent clones of each overexpression strains.
2.7 Precursor addition and FK506 fermentation
Disodium malonate was selected as a precursor which was added at the 4th day of fermentation of Streptomyces sp. CC01 and Streptomyces sp. C5140 at a final concentration of 5 mM according to the previous research.30 Samples were collected from the 5th day of fermentation, and the fermentation and detection procedures were the same as described in Materials and methods (Section 2.5 and 2.6).2.8 RNA extraction and quantitative real-time RT-PCR analysis
The expression levels of tsuR1, tsuS2, fkbG, fkbH, fkbI, tcsA, tcsB, tcsD, fkbQ and fkbN of the transformants were compared by quantitative real-time RT-PCR (qRT-PCR) analysis. Primers of tsuR1, tsuS2, fkbG, fkbH, fkbI, tcsA, tcsB, tcsD, fkbQ and fkbN used for qRT-PCR were listed in Table S2.† Primers of tsuR1 and tsuS2 were designed according to the information published on NCBI (GenBank FR773992 (ref. 25)). Total RNAs were isolated from cultures grown in main medium at 24, 48 and 72 h, respectively. The RNA samples were treated using gDNA eraser (Takara) to remove genomic DNA. Synthesis of cDNA was conducted using PrimeScript™ RT reagent kit (Takara) under the instruction of the manufacturer, and subsequent qRT-PCR analysis was performed by Corbett Rotor-Gene 3000 Real Time DNA Detection RT-PCR Phenix Therm (Sydney, Australia). hrdB gene was used as an internal control. RT-PCR experiments were performed in triplicate, using RNA samples from three independent clones of each overexpression strains.3 Results
3.1 Sequence analysis of SARPs
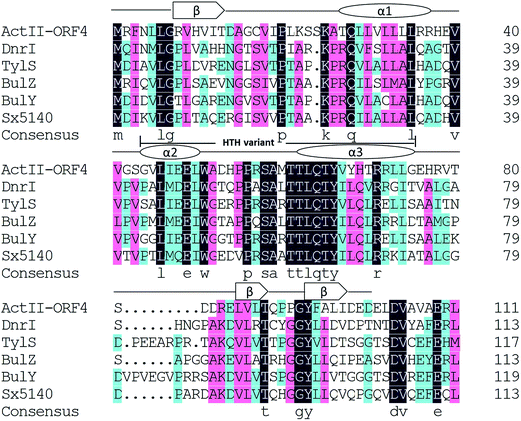
Sx5140 was previously identified in the genome of S. xinghaiensis in our lab as a novel SARP. We are curious on its effect on antibiotic production, and therefore attempted the overexpression of this gene in FK506 producer Streptomyces sp. KCCM11116P. During this study, the partial genomic sequences of a FK506 producer S. tsukubaensis NRRL18448 were available.4,24 Other two putative SARPs, the locations of which are approximate to each other are annotated, we thus amplified the genes encoding these two SARPs from Streptomyces sp. KCCM11116P. Sequencing analysis showed that the corresponding sequences are the same as those in S. tsukubaensis NRRL18488, and we thus also adopted the names of these two genes as bulZ and bulY according to the literature.25 Many other putative regulators that display high identities (51–81%) with Sx5140 were revealed to be present in various Streptomyces species in GenBank database, but many of them have not been characterized so far. Sequences alignment of three well-studied SARP family regulatory proteins, DnrI,18 ActII-ORF4 (ref. 16) and TylS20 with BulZ, BulY and Sx5140 revealed similar predicted secondary structure as well as the HTH variant,15 which was marked in Fig. 1. It is clear that BulZ, BulY and Sx5140 have the typical characteristics of SARP family proteins.3.2 Overexpression of SARPs and analysis of FK506 production
In order to find out whether Sx5140 can act as a positive regulator in FK506 production, we introduced its encoding gene into Streptomyces sp. KCCM11116P using a high-copy-number plasmid pWHM3,31 and the FK506 titer was detected. The resultant strain was named as Streptomyces sp. C5140 (Sx5140 overexpression strain), and Streptomyces sp. CC01 containing the empty vector of pWHM3 was used as the control strain. After finding the positive effect of Sx5140 on FK506 production, other two strains, Streptomyces sp. CC02 (bulZ overexpression strain) and Streptomyces sp. CC03 (bulY overexpression strain) were also constructed, and we then compared FK506 production using these three overexpression strains.When cell growth was evaluated, it was found that biomass accumulation of strains CC02, CC03 and C5140 was the same as that in the control strain CC01 (Fig. 2a). The titers of FK506 of these four strains in mycelia and supernatant were both examined, and the results were shown in Fig. 2. In all the three overexpression strains, the titers of FK506 were higher in mycelia than those in supernatant. It is very clear that no matter in mycelia or in supernatant, FK506 production in strains CC02 and CC03 containing the endogenous SARPs encoding genes was higher than that of the control strain CC01. The most significantly enhanced productivity of FK506 was noticed in strain CC02 (365.59 mg L−1), where the production titer was 1.6-fold of that of the control strain (227.99 mg L−1). Overexpression of the exogenous SARP gene Sx5140 resulted in significant elevation of FK506 production before the 3rd day, after which the production level remained almost constant.
On the other hand, overexpression of bulZ, bulY and Sx5140 resulted in a dramatic change in the initiation of FK506 production and FK506 productivity, and the FK506 productivity in each day of CC02, CC03 and C5140 was higher or similar with that of the control strain CC01. The most significantly enhanced productivity of FK506 was noticed in strain C5140 on the mycelia on the 3rd day, where the productivity of strain C5140 was 2.81-fold of that of the control strain. However, in the later fermentation stages, the higher productivity of FK506 in strain C5140 than the control strain was not retained. We thus confirmed that overexpression of bulZ and bulY in Streptomyces sp. KCCM11116P significantly enhanced the titer of FK506, and overexpression of Sx5140 gene in Streptomyces sp. KCCM11116P resulted in early initiation of FK506 production as well as improved the productivity of FK506, especially at 3rd day.
We assumed that the limitation of precursor supply at further fermentation stage may result in the low titer of FK506 in strain C5140; therefore, precursor addition was performed. The results were presented in Fig. S1.† FK506 titer in both the control strain and strain C5140 was higher when disodium malonate was added, which indicated that disodium malonate addition was indeed beneficial for FK506 production. However, no increase in FK506 production was observed in strain C5140 after precursor addition when compared with the control, which indicated that the limitation of FK506 production fermentation in the later days in Streptomyces sp. C5140 was not the lack of precursor. Further investigation is needed to study the underlying mechanism.
3.3 qRT-PCR analysis of key genes
bulZ and bulY are located in the gene cluster of gamma-butyrolactone in Streptomyces tsukubaensis NRRL18488T,25 on the other hand, we did not find similar organization of genes near the location of Sx5140 in the genome of S. xinghaiensis. Gamma-butyrolactone and its analogue play important roles on regulation of secondary metabolism in streptomycetes,32–34 and previous studies showed that genes involved in the gamma-butyrolactone synthesis had different effects on secondary metabolites production in Streptomyces. For example, in S. lavendulae FRI-5, there are two SARP family regulators involved in the gamma-butyrolactone cluster (IM-2 gene cluster), namely FarR3 and FarR4, which showed different contributions to the regulation of secondary metabolism. FarR3 positively controls the biosynthesis of indigoidine, and FarR4 negatively controls the biosynthesis of a gamma-butyrolactone signaling molecule IM-2.35 In S. tsukubaensis NRRL18488T, tsuR1 (also named bulR1) is a receptor involved in the gamma-butyrolactone cluster, and previous study showed that it plays a positive role on FK506 production; tsuS2 (also named bulS2) encodes a putative gamma-butyrolactone synthetase, but no involvement of this gene on FK506 production was revealed.25 FkbN is a positive regulator in the FK506 biosynthetic gene cluster.6,7 We therefore determined the expression levels of tsuR1 and fkbN by qRT-PCR analysis. fkbG, fkbH, fkbI and tcsA, tcsB, tcsD are responsible for precursor biosynthesis: fkbG, fkbH and fkbI are involved in methoxymalonyl-ACP biosynthesis;36 whereas tcsA, tcsB and tcsD are involved in allymalonyl-CoA biosynthesis.37 fkbQ encodes a type II thioesterase in the FK506 biosynthetic gene cluster. The RNA quantification results were shown in Fig. 3. In the control strain Streptomyces sp. CC01, the transcriptional levels of tsuR1 were down-regulated at 24 h, 48 h and 72 h, consecutively, in contrast, the transcriptional levels of all other genes were up-regulated at 48 h but down-regulated at 72 h in the control strain. In contrast, the transcriptional levels of all of these genes were up-regulated at 48 h and 72 h in all the SARPs overexpressing strains of Streptomyces sp. CC02, Streptomyces sp. CC03 and Streptomyces sp. C5140, and significant increase of tsuR1 expression was observed at 72 h in Streptomyces sp. CC02 and Streptomyces sp. CC03. These results verified that overexpression of Sx5140, BulZ and BulY in Streptomyces sp. KCCM11116P led to the variation of the transcriptional levels of key genes involved in precursor biosynthesis. Transcription level changes of tsuS1 and tsuS2, which encode putative gamma-butyrolactone synthetases, were also detected. However, due to the low level of transcription of tsuS1 in all the three time points and tsuS2 at 72 h, only data of tsuS2 at 24 h and 48 h were available. The expression of tsuS2 was the highest at 48 h in strain C5140, but it is possible that there is not positive correlation of this gene with FK506 production due to the low expression of this gene (Fig. S2†).4 Discussion
Manipulation of regulatory genes for antibiotic biosynthesis has been proved to be effective to stimulate antibiotic production. However, most current studies are focusing on the innate regulatory proteins. With the development of systems biology and synthetic biology, it is possible to select and design a variety of regulatory proteins for antibiotic overproduction. We show here that the exogenous SARP Sx5140 exerts regulatory role in FK506 producer, and through this clue we further found two innate SARPs that can improve FK506 production. Exploration of regulatory function of unknown genes using heterologous regulatory genes is a novel and effective method to improve secondary metabolite production by genetic engineering. We observed different effect of Sx5140 from the endogenous SARPs (BulZ and BulY), and we deduce that Sx5140 may have different binding properties to the target genes from those of BulZ and BulY. This is the first study on the effect of SARP family regulator on FK506 overproduction. These results provide alternative ways to explore more regulatory proteins to improve the production of FK506 as well as other useful secondary metabolites in Streptomyces.BulZ and BulY are two SARP family regulators involved in the gamma-butyrolactone biosynthetic gene cluster in S. tsukubaensis NRRL18488T,25 we supposed that these two genes were also involved in the gamma-butyrolactone biosynthesis and regulation in Streptomyces sp. KCCM11116P. BulZ and BulY are also similar to FarR3 and FarR4, which are two SARPs involved in the gamma-butyrolactone cluster in S. lavendulae FRI-5.35 Based on the previous finding that BulR1 had the positive role on FK506 production in the S. tsukubaensis NRRL18488T,25 we deduced that overexpression of BulZ and BulY exerts its stimulating effect through up-regulation of tsuR1 expression. However, we did not find positive relationship of tsuS2 with the function of BulZ and BulY. Further studies are required to further utilize the combinational overexpression of multiple regulatory genes to enhance FK506 production. The reason that overexpression of Sx5140 did not result in higher final production titer may be due to the failure to ensure high tsuR1 expression especially at later fermentation stage (72 h). On the other hand, although high level tsuR1 was expressed in bulY overexpressing strain Streptomyces sp. CC03, the production titer of this strain was lower than that of Streptomyces sp. CC02, which indicated that tsuR1 was not the only factor that determined high FK506 production level. fkbG, fkbH, fkbI, tcsA, tcsB, tcsD and fkbQ genes were all up-regulated in the three SARP-overexpressing strains in different time points, which implied that the enhancement of FK506 production in these strains at least partially attributes to the up-regulation of precursor biosynthetic genes. Further studies are required to unveil the underlying mechanisms.
In the previous study, overexpression of the putative extracytoplasmic function (ECF) sigma (σ) factor FujE encoding gene in Streptomyces sp. KCCM11116P resulted in about 2.87-fold FK506 production level of the control strain in R2YE medium, but the production titer was very low (less than 25 mg L−1).9 Although moderate enhancement of FK506 production titer was reported in this study (about 1.6-fold of that of the control strain), much higher production titer was achieved (up to 365.59 mg L−1). We assume that the production medium used in our current study enabled the exploration of the full potential of the wild-type strain, therefore less difference was observed between the SARPs overexpressing strains and the control strain. On the other hand, when fkbN was overexpressed together with the FK506 gene cluster in S. coelicolor M1146, about 5-fold production titer (about 5 mg L−1) was achieved compared to the heterologous strain without fkbN overexpression (about 1 mg L−1).8 FkbN situates inside the FK506 gene cluster and plays a positive role in FK506 production.6 The direct action of fkbN function may be the one of the reasons that its overexpression increased FK506 production significantly. In another report, the mutant strain of Streptomyces sp. RM7011 produced 94.24 mg L−1 FK506, 11.63-fold higher than that that of the wild-type strain,10 which indicated that application of a combined approach (random mutagenesis and metabolite engineering to increase precursor supply) to enhance FK506 production is efficient. Combination of SARPs overexpression with other metabolic engineering methods to enhance FK506 production may be necessary to further improve the production of FK506. The positive role of the endogenous and exogenous SARPs on FK506 production reported in this study benefits further exploration of other regulatory genes that exert control on FK506 biosynthesis.
5 Conclusion
Overexpression of three SARPs (BulZ, BulY and Sx5140) enhanced production titer of FK506, and the highest production level of 365.59 mg L−1 was achieved, which is 1.6-fold of that of the control strain. This is the first study on the effect of SARP family regulator on FK506 overproduction. Different expression profiling of tsuR1 which is involved in gamma-butyrolactone biosynthesis, and fkbN, the positive regulator of FK506 biosynthesis, together with the genes of fkbG, fkbH, fkbI, tcsA, tcsB and tcsD that are involved in the FK506 precursor biosynthesis were detected in the SARPs overexpressing strains. These results provide alternative ways to explore more regulatory proteins to improve the production of useful secondary metabolites in Streptomyces.Acknowledgements
This work was supported by a grant of “Cooperative Research Program for Agriculture Science & Technology Development (Project no. PJ01128702)”, Rural Development Administration, Republic of Korea.References
- T. Kino, H. Hatanaka, M. Hashimoto, M. Nishiyama, T. Goto, M. Okuhara, M. Kohsaka, H. Aoki and H. Imanaka, J. Antibiot., 1987, 40, 1249–1255 CrossRef CAS.
- W. H. Parsons, N. H. Sigal and M. J. Wyvratt, Ann. N. Y. Acad. Sci., 1993, 685, 22–36 CrossRef CAS PubMed.
- R. J. Keenan, H. Konishi, A. Kawai, I. L. Paradis, D. R. Nunley, A. T. Iacono, R. L. Hardesty, R. J. Weyant and B. P. Griffith, Ann. Thorac. Surg., 1995, 60, 580–585 CrossRef CAS.
- C. Barreiro and M. Martínez-Castro, Appl. Microbiol. Biotechnol., 2014, 98, 497–507 CrossRef CAS PubMed.
- S. Mo, D. H. Kim, J. H. Lee, J. W. Park, D. B. Basnet, Y. H. Ban, Y. J. Yoo, S. W. Chen, S. R. Park and E. A. Choi, J. Am. Chem. Soc., 2010, 133, 976–985 CrossRef PubMed.
- S. Mo, Y. J. Yoo, Y. H. Ban, S. K. Lee, E. Kim, J. W. Suh and Y. J. Yoon, Appl. Environ. Microbiol., 2012, 78, 2249–2255 CrossRef CAS PubMed.
- D. Goranovič, M. Blažič, V. Magdevska, J. Horvat, E. Kuščer, T. Polak, J. Santos-Aberturas, M. Martínez-Castro, C. Barreiro and P. Mrak, BMC Microbiol., 2012, 12, 238 CrossRef PubMed.
- A. C. Jones, B. Gust, A. Kulik, L. Heide, M. J. Buttner and M. J. Bibb, PLoS One, 2013, 8, e69319 CAS.
- S. K. Lee, S. H. Yang, C. M. Kang, S. Mo and J. W. Suh, Can. J. Microbiol., 2014, 60, 363–369 CrossRef CAS PubMed.
- S. Mo, S. K. Lee, Y. Y. Jin, C. H. Oh and J. W. Suh, Appl. Microbiol. Biotechnol., 2013, 97, 3053–3062 CrossRef CAS PubMed.
- M. J. Bibb, Curr. Opin. Microbiol., 2005, 8, 208–215 CrossRef CAS PubMed.
- J. F. Martín and P. Liras, Curr. Opin. Microbiol., 2010, 13, 263–273 CrossRef PubMed.
- N. P. Niraula, S. H. Kim, J. K. Sohng and E. S. Kim, Appl. Microbiol. Biotechnol., 2010, 87, 1187–1194 CrossRef CAS PubMed.
- H. Zhu, S. K. Sandiford and G. P. Wezel, J. Ind. Microbiol. Biotechnol., 2014, 41, 371–386 CrossRef CAS PubMed.
- A. Wietzorrek and M. Bibb, Mol. Microbiol., 1997, 25, 1181–1184 CrossRef CAS.
- M. A. Fernández-Moreno, J. Caballero, D. A. Hopwood and F. Malpartida, Cell, 1991, 66, 769–780 CrossRef.
- E. Takano, H. Gramajo, E. Strauch, N. Andres, J. White and M. Bibb, Mol. Microbiol., 1992, 6, 2797–2804 CrossRef CAS PubMed.
- P. J. Sheldon, S. B. Busarow and C. R. Hutchinson, Mol. Microbiol., 2002, 44, 449–460 CrossRef CAS.
- K. J. Stutzman-Engwall, S. Otten and C. R. Hutchinson, J. Bacteriol., 1992, 174, 144–154 CAS.
- N. Bate, D. R. Bignell and E. Cundliffe, Mol. Microbiol., 2006, 62, 148–156 CrossRef CAS PubMed.
- X. Q. Zhao, W. J. Li, W. C. Jiao, Y. Li, W. J. Yuan, Y. Q. Zhang, H. P. Klenk, J. W. Suh and F. W. Bai, Int. J. Syst. Evol. Microbiol., 2009, 59, 2870–2874 CrossRef CAS PubMed.
- X. Q. Zhao and T. H. Yang, J. Bacteriol., 2011, 193, 5543 CrossRef CAS PubMed.
- C. Barreiro, C. Prieto, A. Sola-Landa, E. Solera, M. Martínez-Castro, R. Pérez-Redondo, C. García-Estrada, J. F. Aparicio, L. T. Fernández-Martínez and J. Santos-Aberturas, J. Bacteriol., 2012, 194, 3756–3757 CrossRef CAS PubMed.
- M. Blažič, A. Starcevic, M. Lisfi, D. Baranasic, D. Goranovič, Š. Fujs, E. Kuščer, G. Kosec, H. Petković and J. Cullum, Appl. Environ. Microbiol., 2012, 78, 8183–8190 CrossRef PubMed.
- Z. Salehi-Najafabadi, C. Barreiro, A. Rodríguez-García, A. Cruz, G. E. López and J. F. Martín, Appl. Microbiol. Biotechnol., 2014, 98, 4919–4936 CrossRef CAS PubMed.
- E. t. Shirling and D. Gottlieb, Int. J. Syst. Bacteriol., 1966, 16, 313–340 CrossRef PubMed.
- S. Joseph and W. David, Molecular Cloning: A Laboratory Manual, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York, 2001 Search PubMed.
- T. Kieser, M. J. Bibb, M. J. Buttner, K. F. Chater and D. A. Hopwood, Practical Streptomyces genetics, John Innes Foundation, Norwich, 2000 Search PubMed.
- S. Mo, Y. H. Ban, J. W. Park, Y. J. Yoo and Y. J. Yoon, J. Ind. Microbiol. Biotechnol., 2009, 36, 1473–1482 CrossRef CAS PubMed.
- W. J. Du, D. Huang, M. L. Xia, J. P. Wen and M. Huang, J. Ind. Microbiol. Biotechnol., 2014, 41, 1131–1143 CrossRef CAS PubMed.
- J. Vara, M. Lewandowska-Skarbek, Y. G. Wang, S. Donadio and C. Hutchinson, J. Bacteriol., 1989, 171, 5872–5881 CAS.
- N. H. Hsiao, S. Nakayama, M. E. Merlo, M. Vries, R. Bunet, S. Kitani, T. Nihira and E. Takano, Chem. Biol., 2009, 16, 951–960 CrossRef CAS PubMed.
- E. Takano, Curr. Opin. Microbiol., 2006, 9, 287–294 CrossRef CAS PubMed.
- G. Y. Tan, L. Q. Bai and J. J. Zhong, Biotechnol. Bioeng., 2013, 110, 2984–2993 CrossRef CAS PubMed.
- Y. N. Kurniawan, S. Kitani, A. Maeda and T. Nihira, Appl. Microbiol. Biotechnol., 2014, 98, 9713–9721 CrossRef CAS PubMed.
- Y. A. Chan, M. T. Boyne, A. M. Podevels, A. K. Klimowicz, J. Handelsman, N. L. Kelleher and M. G. Thomas, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 14349–14354 CrossRef CAS PubMed.
- S. Mo, D. H. Kim, J. H. Lee, J. W. Park, D. B. Basnet, Y. H. Ban, Y. J. Yoo, S. W. Chen, S. R. Park and E. A. Choi, J. Am. Chem. Soc., 2010, 133, 976–985 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra15038d |
| This journal is © The Royal Society of Chemistry 2015 |