Effect of iron acetylacetonate on the crosslink structure, thermal and flammability properties of novel aromatic diamine-based benzoxazines containing cyano group
Hongqiang Yana,
Huaqing Wangb,
Jie Chenga,
Zhengping Fang*a and
Hao Wangc
aLab of Polymer Material and Engineering, Ningbo Institute of Technology, Zhejiang University, Ningbo 315100, China. E-mail: zpfang@zju.edu.cn; Fax: +86 574 88220132; Tel: +86 574 88220132
bZhejiang Textile & Fashion Technology Collage, Ningbo 315211, China
cCentre of Excellence in Engineered Fibre Composites (CEEFC), University of Southern Queensland, Toowoomba, Queensland 4350, Australia
First published on 28th January 2015
Abstract
Iron acetylacetonate (Fe(AcAc)3) was chosen as the catalyst for a novel aromatic diamine-based benzoxazine, containing cyano group (BAPBACP). Its effect on the curing process, thermal and flammability properties of BAPBACP were investigated. The results indicated that without Fe(AcAc)3, the ring-opening polymerization of the BAPBACP monomer occurred and an arylamine Mannich bridge structure was formed at the low curing temperature stage; and then the cyclotrimerization of the cyano group followed at the high curing temperature stage, but the cyano group was not fully cyclotrimerized even after curing at 350 °C for 0.5 h. The addition of 3.5% Fe(AcAc)3 speeded up the curing reaction and the cyano group was fully cyclotrimerized at 350 °C. Thermogravimetric analysis and microscale combustion calorimetry results showed that the poly(BAPBACP) resins possess excellent thermal and flammability properties due to the existence of the arylamine Mannich bridge structure and triazine ring in their crosslinked structure.
1. Introduction
Benzoxazine resins have attracted increasing attention in the past decade due to their excellent high temperature stability, low water absorption, good dielectric properties and structure design flexibility, in addition to their zero shrinkage or only slight expansion upon curing.1–4 These good properties give them huge potential in industrial applications such as high speed printed circuit boards, aerospace structural composites.5,6 With the rapid development of aerospace, microelectronics and energy industries, there is an urgent requirement to further improve the properties of benzoxazine resins such as heat resistance, flame retardance, toughness, low dielectric constant. Therefore, the further enhancement of the thermal stability and flame retardancy of polybenzoxazines has become one of the research focuses.In general, the introduction of additional functional groups into monomers to prepare copolymers and polymer alloys is widely used to achieve high performance in polybenzoxazines.2,4 For example, benzoxazines, which are usually derived from phenols/biphenols, monoamines and formaldehyde, have been prepared by introducing allyl, acetylene, nitrile, maleimide and aldehyde groups to the main chain or terminal position.4,7–10 The copolymerization of benzoxazine with another reactive comonomer has been developed, such as urethane–benzoxazine, benzoxazine–epoxy, benzoxazine–cyanate and benzoxazine–cyanate–epoxy copolymers,11–14 but the resulting systems often exhibit higher curing temperature and viscosity than the neat polybenzoxazines.7,15
Due to the structure design flexibility of benzoxazine resins, another simple and attractive approach is to synthesize aromatic diamine-based benzoxazines, which are derived from aromatic diamines, monophenols and formaldehyde. Recently, a series of novel monomers have been successfully synthesized by using high boiling point nonpolar solvent or a three-step synthetic method, with increased thermal stability compared to the conventional polybenzoxazines due to the nitrogen linkage to other repeating units.16,17 However, to date, very limited success in preparing this type of benzoxazine has been achieved, mainly because of the difficulty of this benzoxazine ring-forming reaction and the unknown structure–property relationship. In our recent study,18 three types of novel aromatic diamine-based benzoxazines, containing a cyano group have been designed and synthesized by the reaction of 4-hydroxybenzonitrile, paraformaldehyde, and pre-synthesized diamine compounds. These novel benzoxazine resins show good thermal stability, with especially high char residue. However, it seems that the cyano group does not cure under normal curing conditions because the cyclotrimerization of the cyano group is very difficult. To fully understand and control the curing process of this new thermoset, it is necessary to study the curing kinetics of the thermoset in detail and the effect of catalyzers on the curing reaction of the cyano group and benzoxazine resin properties.
The purpose of this research is to study the curing process of 3,3′-(((propane-2,2-diylbis(4,1-phenylene))bis(oxy))bis(4,1-phenylene))bis(3,4-dihydro-2H-benzo[e][1,3]oxazine-6-carbonitrile) (BAPBACP) with and without catalysts. For the choice of catalyst, many types of catalysts have been studied for the curing reaction of benzoxazine resins.19–21 Gu et al.21 found that benzoxazine resins could partially undergo ring-opening polymerization at a low temperature in the presence of FeCl3 and generate some oligomers, containing N,O-acetal-type bridge structures, which can delay the degradation of benzoxazine resins; but, the inorganic metal catalyst of FeCl3 cannot be dissolved in benzoxazine resins easily, resulting in very low catalytic efficiency. In order to improve the efficiency of catalysts, metal organic compounds are added to dissolve in benzoxazine monomer to effectively catalyze resin curing. Moreover, metal organic compounds are also high efficient catalysts for cyano groups.22–24 In this study, iron acetylacetonate (Fe(AcAc)3) is chosen as the catalyst for the BAPBACP monomer system; and the effects of Fe(AcAc)3 on the complex curing process and the thermal and flammability properties of BAPBACP resin are determined by using DSC, FTIR, TG and MCC.
2. Experimental
2.1. Materials
The synthesis of BAPBACP monomer has been described elsewhere.18 The structure of BAPBACP is shown in Scheme 1. Iron acetylacetonate (Fe(AcAc)3; 99%) was used as received from J&K Chemical Co. Ltd. (Shanghai, China). All solvents used were of reagent grade.2.2. Sample preparation
The required amount of iron acetylacetonate (Fe(AcAc)3) was added to the preselected weight of BAPBACP dissolved in chloroform, stirred for 5 min, and the solvent was evaporated in vacuum at room temperature. The mixture was pulverized to obtain a homogeneous mixture. The BAPBACP monomer and its mixture containing 3.5% Fe(AcAc)3 were cured in an air oven by a stagewise heating process: 170 °C for 2 h, 190 °C for 2 h and 210 °C for 2 h. The sample obtained is denoted as poly(BAPBACP)-210. Poly(BAPBACP)-210 resin was further cured in a muffle furnace at 280 °C for 0.5 h (denoted as poly(BAPBACP)-280), then at 350 °C for 0.5 h (denoted as poly(BAPBACP)-350). The cured resins were measured by thermogravimetric analysis (TG) and microscale combustion calorimetry (MCC).2.3. Measurement
DSC measurements were performed with a Netzsch 200 P C supported with a Netzsch TASC 414-5 computer for data acquisition. DSC was calibrated with high purity indium, and measurements were conducted under a nitrogen flow of 20 cm3 min−1. In DSC experiments, all the samples were subjected to a dynamic DSC scan from 50 to 400 °C at heating rate of 10 °C min−1. In situ FTIR studies were performed using a Thermo Nicolet iS10 FT-IR spectrophotometer (Nicolet) equipped with a temperature-controlled sample holder that allows for the in situ analysis of the curing reaction. Thin resin film was prepared by the resin being dissolved in solvent and casted on NaCl cells. The samples were then seared in the preheated sample holder for the designed stagewise heating process (170 °C for 2 h, 190 °C for 2 h, 210 °C for 2 h, 280 °C for 0.5 h and 350 °C for 0.5 h). The spectra were obtained at the end of every isothermal stage. The C–H vibration of 1,2,4-trisubstituted benzene ring at 1500 cm−1 was used as the reference peak, which should not be changed during the curing reaction. Because the area of peak is directly proportional to the concentration, the normalized concentration of cyano vibrations at any temperature can be calculated fromwhere A(T) is the absorbance of cyano vibrations at temperature T; A(T)1500 is the absorbance of 1,2,4-trisubstituted benzene ring at temperature T; A(0) is the initial absorbance of cyano vibrations and A(0)1500 is the initial absorbance of 1,2,4-trisubstituted benzene ring; and C(T) is the fraction of unreacted cyano group. TG analysis was carried out using a TGA 209 F1 Thermal Analyzer (Netzsch, Germany) to study the thermal degradation behavior of the cured resins. Samples were heated from room temperature to 950 °C at a heating rate of 20 °C min−1 in nitrogen with a flow rate of 40 cm3 min−1. Microscale combustibility experiments were conducted using an MCC-2 MCC (Govmark, USA). 5 mg sample was heated to 750 °C at a heating rate of 1 °C s−1 in a mixed stream of oxygen and nitrogen flowing at 20 and 80 cm3 min−1, respectively.
3. Result and discussion
3.1. DSC measurements of BAPBACP resin systems
DSC was carried out to study the curing behavior of the BAPBACP monomer systems. Fig. 1 shows the typical nonisothermal DSC thermograms; and the results are summarized in Table 1. | ||
| Fig. 1 The DSC curves of the BAPBACP monomers (a) neat BAPBACP monomer; (b) BAPBACP monomer with 3.5% Fe(AcAc)3. | ||
| Tm/°C | Tp1/°C | ΔH1/J g−1 | Tp2/°C | ΔH2/J g−1 | |
|---|---|---|---|---|---|
| Neat BAPBACP monomer | 185.4 | 240.1 | 246.4 | 341.4 | 61.6 |
| BAPBACP monomer, including 3.5% Fe(AcAc)3 | 173.7 | 218.1 | 72.3 | 350.6 | 46.0 |
The DSC curves given in Fig. 1 show a clear two-stage curing for the two BAPBACP monomer systems. The first stage was the endothermic melting of the BAPBACP monomer. Adding 3.5%Fe(AcAc)3 lowered the melting temperature from 185.4 °C to 173.7 °C. The second stage was the exothermic curing reactions, with a sharp exothermic peak in a temperature range of 210–290 °C with a maximum at 240.1 °C (Tp1), and a small exothermic peak in a temperature range of 290–390 °C with a maximum at 341.4 °C (Tp2), which were attributed to the ring-opening polymerization of benzoxazine and homo-polymerization of the cyano group, respectively. When 3.5% Fe(AcAc)3 was added as catalyst, the first curing exotherm shifted to a lower temperature, as observed from a systematic drift in the curing characteristics, such as peak temperature (Tp1) from 240.1 °C to 218.1 °C, and its ΔH1 value also decreased from 246.4 J g−1 to 72.3 J g−1. However, the second curing exotherm was little altered, and the ΔH2 value only decreased from 61.6 J g−1 to 46.0 J g−1. The first curing reaction of BAPBACP monomer at low temperature stage was significantly catalyzed by Fe(AcAc)3, but the second curing reaction of BAPBACP monomer was almost not affected. As a transition metal salt, Fe(AcAc)3 is an effective catalyst for polymerization of BAPBACP monomer even in a small quantity.
3.2. FTIR analysis of BAPBACP monomer systems
The different curing behaviors were caused probably by different curing mechanisms. To further study the curing behavior of BAPBACP monomer systems with and without Fe(AcAc)3 and their crosslinked structures, the FTIR spectra of BAPBACP monomer systems cured at various temperatures were conducted.Fig. 2 shows the FTIR spectra of the neat BAPBACP monomer at different cure temperatures. The characteristic absorption wavenumbers for the FTIR spectra are listed in Table 2. At the initial reaction stage (BAPBACP curve in Fig. 2), there were evident characteristic peaks of the cyano group (2223 cm−1) and out-of-plane C–H of the oxazine ring (934 cm−1). After curing at 170 °C for 2 h, the 934 cm−1 out-of-plane C–H of oxazine ring peak disappeared completely, confirming the ring-opening polymerization of the oxazine ring. However, the absorption band at approximately 1238 cm−1 did not disappear completely, which was probably due to the asymmetric stretching vibration of aryl ether linkages, which overlapped with that of C–O–C for the oxazine ring.25 Furthermore, the intensity of the absorption band at 1500 cm−1, assigned to 1,2,4-trisubstituted benzene, had little change during the curing, indicating that the benzene ring in the structure of the BAPBACP monomer did not alter during the ring-opening process. The band at 1480 cm−1, assigned to 1,2,4,6-tetrasubstituted benzene, could not be observed. Moreover, the intensity of the band at 1118 cm−1 assigned to C–N–C bond weakened gradually. These results indicated that the main crosslinked structure was arylamine Mannich bridge, rather than phenolic Mannich bridge. At curing temperatures below 210 °C, the intensity of the band at 2223 cm−1 assigned to the cyano group weakened slowly, and the band at 1604 cm−1 assigned to the triazine groups (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–C) could be observed, indicating that some cyano groups of the BAPBACP monomer had been cyclotrimerized to form a triazine structure. This result demonstrated that the cyclotrimerization of the cyano group was slower than the ring-opening polymerization of the oxazine ring. At the same time, new absorption was observed at 1680 cm−1 and assigned to 1,2-bisquinone compound, which might be attributed to the C–N stretching of a Schiff base byproduct formed in the ring-opening reaction.13,26,27 When the curing temperatures were above 210 °C, the intensity of the band of the cyano group decreased obviously, meaning the cyclotrimerization of the cyano group was prompted at the high curing temperature stage. The band at 2223 cm−1 did not disappear completely after curing at 350 °C for 0.5 h. This indicated that the formation of the triazine structure was very difficult and that the cyano group was not cyclotrimerized completely. The crosslinked structure of poly(BAPBACP) resin is shown in Scheme 2.
N–C) could be observed, indicating that some cyano groups of the BAPBACP monomer had been cyclotrimerized to form a triazine structure. This result demonstrated that the cyclotrimerization of the cyano group was slower than the ring-opening polymerization of the oxazine ring. At the same time, new absorption was observed at 1680 cm−1 and assigned to 1,2-bisquinone compound, which might be attributed to the C–N stretching of a Schiff base byproduct formed in the ring-opening reaction.13,26,27 When the curing temperatures were above 210 °C, the intensity of the band of the cyano group decreased obviously, meaning the cyclotrimerization of the cyano group was prompted at the high curing temperature stage. The band at 2223 cm−1 did not disappear completely after curing at 350 °C for 0.5 h. This indicated that the formation of the triazine structure was very difficult and that the cyano group was not cyclotrimerized completely. The crosslinked structure of poly(BAPBACP) resin is shown in Scheme 2.
| Chemical group | Absorption wavenumbers/cm−1 |
|---|---|
| Out-of-plane C–H | 934 |
| C–N–C bond | 1118 |
| C–O–C bond | 1238 |
| 1,2,4-Trisubstituted benzene | 1500 |
| Triazine ring | 1604 |
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond N bond |
1680 |
| Cyano group | 2223 |
Fig. 3 shows the FTIR spectra of the BAPBACP monomer system with 3.5% Fe(AcAc)3 at different curing temperatures; compared with Fig. 2, they are similar. The C(T)s of the cyano group for the BAPBACP monomer systems are shown in Fig. 4. The change of C(T) of the cyano group in the catalyzed system beyond 210 °C was quicker than that in the neat system, indicating that the cure reaction of the cyano group was speeded up, evidently as a result of the existence of Fe(AcAc)3. After curing at 350 °C for 0.5 h, the band at 2223 cm−1 had disappeared completely, indicating that with the addition of 3.5% Fe(AcAc)3, the formation of the triazine structure became easier and more triazine groups were formed in the crosslinked structure of poly(BAPBACP) resin. With the existence of phenol hydroxyl, which was generated during the ring-opening polymerization of benzoxazine, the cyano groups had a high tendency for catalysis by metal ions (Fe3+) through coordination with the cyano groups, as shown in Scheme 3. The mechanism involved the formation of a metal complex intermediate with reduced electron density at the carbon atom, which could react with the nucleophilic nitrogen of another cyano group to form triazine and regenerate the catalyst.22,22,28 Additionally the band at 1680 cm−1 disappeared, meaning that the C–N stretching of the Schiff base was not oxidized in the BAPBACP monomer system with 3.5% Fe(AcAc)3.
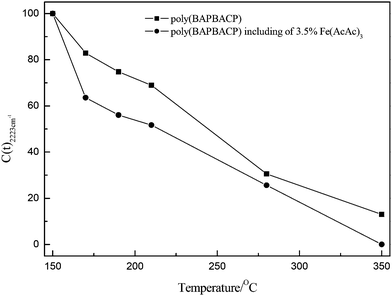 | ||
| Fig. 4 The C(T) variation of the cyano group at 2223 cm−1 for the BAPBACP monomer systems at different curing temperatures. | ||
 | ||
| Scheme 3 Mechanism of catalysis of cure reaction for the BAPBACP monomer system with 3.5% Fe(AcAc)3. | ||
3.3. Thermal properties of poly(BAPBACP) resin
The thermal behavior (thermal stability) of poly(BAPBACP) resins was evaluated using TG under nitrogen atmosphere. The TG curves of poly(BAPBACP) resins and their derivatives (DTG) are shown in Fig. 5 and 6, with the results summarized in Table 3 and 4. The extensional onset temperature (Te) is defined as the onset temperature of the degradation of different poly(BAPBACP) resins. Tmax is the temperature with the maximum mass loss rate, Mass is the mass loss of every decomposition stage, peak value is the maximum mass loss rate of every decomposition stage in DTG curve, and char is the percentage of the char yielded at 800 °C under nitrogen atmosphere. | ||
| Fig. 6 TG curves (a) and DTG curves (b) for poly(BAPBACP) resins with 3.5% Fe(AcAc)3 under nitrogen atmosphere. | ||
| Sample ID | Te (°C) | Tmax1 (°C) | Mass1 (%) | Peak1 value (wt%/°C) | Tmax2 (°C) | Mass2 (%) | Peak2 value (wt%/°C) | Char (%) |
|---|---|---|---|---|---|---|---|---|
| Poly(BAPBACP)-210 | 337.6 | 400.9 | 28.7 | 4.56 | 489.4 | 20.6 | 4.05 | 50.7 |
| Poly(BAPBACP)-280 | 374.3 | 459.3 | 21.2 | 4.35 | 504.8 | 24.6 | 4.73 | 54.2 |
| Poly(BAPBACP)-350 | 457.1 | — | — | — | 507.0 | 25.3 | 4.31 | 68.1 |
| Sample ID | Te (°C) | Tmax1 (°C) | Mass1 (%) | Peak1 value (wt%/°C) | Tmax2 (°C) | Mass2 (%) | Peak2 value (wt%/°C) | Char (%) |
|---|---|---|---|---|---|---|---|---|
| Poly(BAPBACP)-210 | 338.5 | 389.0 | 13.6 | 3.09 | 503.1 | 31.2 | 4.40 | 55.3 |
| Poly(BAPBACP)-280 | 392.8 | — | — | — | 501.1 | 30.6 | 4.02 | 62.8 |
| Poly(BAPBACP)-350 | 464.8 | — | — | — | 515.2 | 27.4 | 2.71 | 72.6 |
As shown in Fig. 5 and Table 3, Te, Tmax1 and Tmax2 of poly(BAPBACP)-210 resin are 337.6, 400.9 and 489.4 °C, respectively, and the char residue at 800 °C is 50.7%. As expected, the poly(BAPBACP) resin showed good thermal stability, and especially a high char residue. In its DTG curve, poly(BAPBACP) resin showed a two-stage weight loss process, which was different from the classic three-stage weight loss process in benzoxazine resin.29,30 As seen from Fig. 2, the main crosslinked structure of poly(BAPBACP) resin was the arylamine Mannich bridge structure rather than the phenolic Mannich bridge structure. Compared with the classic three-stage weight loss process, the first major weight loss event of the aniline moieties of the arylamine Mannich bridge structure was retarded to a higher temperature and overlapped with the second event to form one weight loss event, meaning that the arylamine Mannich bridge structure was more stable than the phenolic Mannich bridge structure. With the increase of curing temperature, the characteristic temperature and char residue of poly(BAPBACP) resin had been improved, especially the poly(BAPBACP)-350 resin. This was mainly because more cyano groups of the BAPBACP monomer had been cyclotrimerized to form more triazine structures with the increase of curing temperature. Because the curing reaction of the cyano group was quicker at higher temperatures, Te and Tmax2 of poly(BAPBACP)-350 resin had been improved to 457.1 and 507.0 °C, and its char residue at 800 °C also increased to 68.1%. The DTG curve of poly(BAPBACP)-350 resin gave only a one-stage weight loss process, centered near 510 °C, and its peak value of the DTG curve also decreased, from 4.73 to 4.31 wt%/°C. Thus, one could conclude that the poly(BAPBACP) resins possessed good thermal stability due to the arylamine Mannich bridge structure and the triazine ring in their crosslinked structure.
Fig. 6 shows the TG thermograms and DTG curves of poly(BAPBACP) resins with 3.5% Fe(AcAc)3, with the detailed data summarized in Table 4. For the poly(BAPBACP)-210 resin, its DTG curve still presented a two-stage weight loss process after adding 3.5% Fe(AcAc)3; however, the first peak value of its DTG curve decreased from 4.56 to 3.09 and its char residue at 800 °C was increased from 50.7 to 55.3%, meaning that the thermal stability of poly(BAPBACP) resins had been improved by the addition of Fe(AcAc)3. As seen from Fig. 4, for the poly(BAPBACP)-210 resin with Fe(AcAc)3, the conversion rate of the cyano group increased from 31.1 to 48.3%, resulting in higher triazine ring content, which was beneficial to the thermal stability of the poly(BAPBACP) resin. For the poly(BAPBACP)-280 and poly(BAPBACP)-350 resins with Fe(AcAc)3, their DTG curves presented only a one-stage weight loss process, Tes and char residues at 800 °C increased markedly and the peak values of their DTG curves decreased. The abovementioned results indicated that the thermal stability of poly(BAPBACP) resins could be improved evidently by the addition of Fe(AcAc)3. This was because the cyclotrimerization of the cyano group was prompted by Fe(AcAc)3 to produce more triazine rings in the structure of poly(BAPBACP) resin. These results illustrated that triazine ring structure played a very important role towards the thermal stability of poly(BAPBACP) resin.
3.4. Flammability properties of poly(BAPBACP) resin
In order to further analyze the correlation between the crosslinked structure and flammability of the poly(BAPBACP) resins, their aerobic pyrolysis and the subsequent reactions between the volatile pyrolysis products and a mixture of nitrogen/oxygen (80/20) gas under high temperatures were simulated by microscale combustibility calorimetry experiments (MCC). The key combustion parameters, including heat release capacity (HR Capacity), total heat release (THR), peak for total heat release rate (PHRR) and temperature for the PHRR (TPHRR, which is a good predictor of flammability), could be obtained. The representative heat release rate curves of the poly(BAPBACP) resins are presented in Fig. 7 and 8, with the detailed data summarized in Table 5 and 6.| Sample ID | HR capacity (J g−1 K−1) | THR (kJ g−1) | PHRR (W g−1) | TPHRR (°C) |
|---|---|---|---|---|
| BAPBACP-210 | 64 ± 1 | 11.7 ± 0.1 | 70.1 ± 1.5 | 411.5 ± 3.2 |
| BAPBACP-280 | 50 ± 2 | 6.5 ± 0.4 | 71.2 ± 2.1 | 520.0 ± 0.8 |
| BAPBACP-350 | 43 ± 3 | 3.4 ± 0.4 | 58.6 ± 2.0 | 520.9 ± 2.3 |
| Sample ID | HR capacity (J g−1 K−1) | THR (kJ g−1) | PHRR1 (W g−1) | TPHRR1 (°C) | PHRR2 (W g−1) | TPHRR2 (°C) |
|---|---|---|---|---|---|---|
| BAPBACP-210 | 57 ± 2 | 10.3 ± 0.3 | 56.5 ± 0.2 | 404.4 ± 1.7 | 63.5 ± 0.6 | 520.1 ± 0.8 |
| BAPBACP-280 | 45 ± 1 | 4.2 ± 0.1 | — | — | 59.5 ± 0.5 | 520.4 ± 1.7 |
| BAPBACP-350 | 38 ± 1 | 2.8 ± 0.1 | — | — | 52.8 ± 2.0 | 526.6 ± 1.4 |
As shown in Fig. 7 and Table 5, there is one wide overlapping exothermic peak from 300 to 650 °C in the MCC curve of the poly(BAPBACP)-210 resin, and its THR, HR capacity and PHRR are 11.7 kJ g−1, 64 J g−1 K−1 and 70.1 W g−1, respectively. Compared to the poly(BAPBACP)-210 resin, the exothermic peak of the poly(BAPBACP)-280 resin is narrowed and retarded to a higher temperature, TPHRR increase remarkably from 411.5 to 520.0 °C and its THR and HR Capacity decreased remarkably to 6.5 kJ g−1 and 50 J g−1 K−1, respectively. Furthermore, poly(BAPBACP)-350 resin showed the lowest value of HR capacity, THR and PHRR, indicating that a triazine ring generated by the cyclotrimerization of a cyano group could retard the combustion of poly(BAPBACP) resin, reducing its combustion speed, burning degree and combustion heat release.
As shown in Fig. 8 and Table 6, after adding 3.5% Fe(AcAc)3, the MCC curve of the poly(BAPBACP)-210 resin presents a two-stage combustibility process, and its THR, HR capacity and PHRRs decrease to 10.3 kJ g−1, 57 J g−1 K−1, 56.5 W g−1 and 63.5 W g−1, respectively. The results further demonstrate that more triazine rings, generated from the cyclotrimerization of cyano groups catalyzed by Fe(AcAc)3, enable a strong hindering of combustibility of the arylamine Mannich bridge structure, leading to a gradual reduction in heat release rate. With increasing curing temperature, the first peak of combustibility of the poly(BAPBACP) resin disappears completely, and its THR, HR capacity and PHRR further decrease. The TPHRR values of all the poly(BAPBACP) resins, except poly(BAPBACP)-210 without Fe(AcAc)3, are almost consistent, signifying that the triazine ring structure confers a crucial effect on the flammability stability of poly(BAPBACP) resin. These results show a similar trend obtained from the TGA result under nitrogen atmosphere.
4. Conclusions
In this study, the effect of Fe(AcAc)3 on the complex curing process, thermal and flammability properties of BAPBACP resin was investigated using DSC, FTIR, TG and MCC. The DSC curves of the BAPBACP monomer systems showed a clear two-stage curing. When 3.5% Fe(AcAc)3 was added, the first curing exotherm shifted to a lower temperature, but the second curing exotherm was little altered. According to the FTIR spectra of the BAPBACP monomer system without catalyst, the ring-opening polymerization of the oxazine ring occurred to generate the arylamine Mannich bridge structure at low curing temperature; the cyclotrimerization of the cyano group was prompted only at a high curing temperature stage and was still not completed after curing at 350 °C for 0.5 h. After adding 3.5% Fe(AcAc)3, the curing procedure of the BAPBACP monomer system did not change, but the cyclotrimerization of the cyano group became easier and was completed after curing at 350 °C for 0.5 h. The TG results showed that Te and Tmax2 of poly(BAPBACP)-350 resin were improved to 457.1 and 507.0 °C, respectively, and its char residue at 800 °C was also heightened to 68.1%. These results indicate that the poly(BAPBACP) resin possesses better thermal stability than the classic benzoxazine resin due to the existence of the arylamine Mannich bridge structure and triazine ring. Moreover, the flammability stability of poly(BAPBACP) resin was retarded remarkably by the arylamine Mannich bridge structure and triazine ring. Thus, the poly(BAPBACP) resins possess excellent thermal and flame retardant properties due to the arylamine Mannich bridge structure and triazine ring in their crosslinked structure. Therefore, these results highlight the importance of controlling the crosslinked structure of the poly(BAPBACP) resins and the role of triazine ring in their thermal and flame retardant properties.Acknowledgements
We gratefully acknowledge the financial supports from the National Natural Science Foundation of China (no. 51103129), Zhejiang Provincial Natural Science Foundation of China (LY14E030006), the Ningbo Natural Science Foundation (no. 2012A610084), and the Open Fund of Zhejiang Provincial Top Key Discipline of New Materials and Process Engineering (20110939 and 20121126).References
- H. Ishida and D. P. Sanders, J. Polym. Sci., Part B: Polym. Phys., 2000, 38, 3289–3301 CrossRef CAS.
- R. Andreu, M. A. Espinosa, M. Galià, V. Cádiz, J. C. Ronda and J. A. Reina, J. Polym. Sci., Part A: Polym. Chem., 2006, 44, 1529–1540 CrossRef CAS.
- N. N. Ghosh, B. Kiskan and Y. Yagci, Prog. Polym. Sci., 2007, 32, 1344–1391 CrossRef CAS PubMed.
- A. Chernykh, T. Agag and H. Ishida, Macromolecules, 2009, 42, 5121–5127 CrossRef CAS.
- X. Liu and Y. Gu, J. Appl. Polym. Sci., 2002, 84, 1107–1113 CrossRef CAS.
- A. Chernykh, J. Liu and H. Ishida, Polymer, 2006, 47, 7664–7669 CrossRef CAS PubMed.
- K. S. Santhosh Kumar, C. P. R. Nair, T. S. Radhakrishnan and K. N. Ninan, Eur. Polym. J., 2007, 43, 2504–2514 CrossRef PubMed.
- H. Qi, H. Ren, G. Pan, Y. Zhuang, F. Huang and L. Du, Polym. Adv. Technol., 2009, 20, 268–272 CrossRef CAS.
- T. Agag and T. Takeichi, J. Polym. Sci., Part A: Polym. Chem., 2006, 44, 1424–1435 CrossRef CAS.
- Q. C. Ran and Y. Gu, J. Polym. Sci., Part A: Polym. Chem., 2011, 49, 1671–1677 CrossRef CAS.
- S. Rimdusit, W. Bangsen and P. Kasemsiri, J. Appl. Polym. Sci., 2011, 121, 3669–3678 CrossRef CAS.
- C. Jubsilp, K. Punson, T. Takeichi and S. Rimdusit, Polym. Degrad. Stab., 2010, 95, 918–924 CrossRef CAS PubMed.
- C. H. Lin, S. J. Huang, P. J. Wang, H. T. Lin and S. A. Dai, Macromolecules, 2012, 45, 7461–7466 CrossRef CAS.
- X. D. Li, Y. Q. Xia, W. L. Xu, Q. C. Ran and Y. Gu, Polym. Chem., 2012, 3, 1629–1633 RSC.
- T. Agag and T. Takeichi, J. Polym. Sci., Part A: Polym. Chem., 2007, 45, 1878–1888 CrossRef CAS.
- T. Agag, L. Jin and H. Ishida, Polymer, 2009, 50, 5940–5944 CrossRef CAS PubMed.
- C. H. Lin, S. L. Chang, C. W. Hsieh and H. H. Lee, Polymer, 2008, 49, 1220–1229 CrossRef CAS PubMed.
- T. Zhang, H. Q. Yan, Z. P. Fang and M. Peng, Chin. J. Polym. Sci., 2013, 31, 1359–1371 CrossRef CAS PubMed.
- Y. X. Wang and H. Ishida, Polymer, 1999, 40, 4563–4570 CrossRef CAS.
- H. Y. Low and H. Ishida, Polym. Degrad. Stab., 2006, 91, 805–815 CrossRef CAS PubMed.
- Q. C. Ran, D. X. Zhang, R. Q. Zhu and Y. Gu, Polymer, 2012, 53, 4119–4127 CrossRef CAS PubMed.
- H. Q. Yan, L. Ji and G. R. Qi, J. Appl. Polym. Sci., 2004, 91, 3927–3939 CrossRef CAS.
- H. Q. Yan, S. Chen and G. R. Qi, Polymer, 2003, 44, 7867 CrossRef PubMed.
- D. Mathew, C. P. R. Nair and K. N. Ninan, J. Polym. Sci., Part A: Polym. Chem., 1999, 37, 1103–1114 CrossRef CAS.
- L. Zhang, H. Peng, J. Sui, C. Soeller, P. A. Kilmartin and J. Travas-Sejdic, J. Phys. Chem. C, 2009, 113, 9128–9134 CAS.
- H. Ishida and D. P. Sanders, Polymer, 2001, 42, 3115–3125 CrossRef CAS.
- J. Dunkers and H. Ishida, J. Polym. Sci., Part A: Polym. Chem., 1999, 37, 1913–1921 CrossRef CAS.
- C. P. R. Nair, C. Gopalakrishnan and K. N. Ninan, Polym. Polym. Compos., 2001, 9, 531–539 CAS.
- H. Y. Low and H. Ishida, J. Polym. Sci., Part B: Polym. Phys., 1998, 36, 1935–1946 CrossRef CAS.
- H. Y. Low and H. Ishida, Polymer, 1999, 40, 4365–4376 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |