In situ crosslinkable hydrogels formed from modified starch and O-carboxymethyl chitosan
Abstract
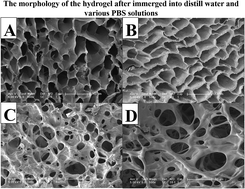
An in situ hydrogel based on oxidation cholesterol starch (OCS) and O-carboxymethyl chitosan (CMCT) that is completely devoid of potentially cytotoxic small molecule cross-linkers and does not require complex manoeuvres or catalysis has been formulated and characterized. The network structure was created by Schiff base formation. The mechanical properties, internal morphology and swelling ability of the injectable hydrogel were examined. Rheological measurements demonstrated that increasing the concentration of the monomer improved the storage modulus. SEM showed that the hydrogel possessed a well-defined porous structure. In addition, the Schiff base reaction was acid sensitive. Under acid conditions, the hydrogel could hydrolyse quickly compared with high pH conditions. Doxorubicin (DOX) was used as a model drug to investigate the control and release properties of the hydrogel. The cytotoxic potential of the hydrogel was determined using an in vitro viability assay with L929 cells as a model and the results revealed that the hydrogel was non-cytotoxic.

 Please wait while we load your content...
Please wait while we load your content...