Microwave synthesis of high luminescent aqueous CdSe/CdS/ZnS quantum dots for crystalline silicon solar cells with enhanced photovoltaic performance
Tong-Tong Xuan,
Jia-Qing Liu,
Hui-Li Li*,
Heng-Chao Sun,
Li-kun Pan,
Xiao-Hong Chen and
Zhuo Sun
Engineering Research Center for Nanophotonics & Advanced Instrument, Ministry of Education, Department of Physics, East China Normal University, Shanghai 200062, China. E-mail: hlli@phy.ecnu.edu.cn
First published on 23rd December 2014
Abstract
Water-dispersed CdSe/CdS/ZnS core/shell/shell quantum dots (QDs) with the highest quantum yield of 25.4% were first synthesized for each component by microwave irradiation. As-prepared QDs do not only possess a large Stokes shift but also exhibit excellent repeatability. They can convert near UV and blue light with lower sensitivity to the Si solar cell to red light at which the solar cell has higher sensitivity. The fabricated CdSe/CdS/ZnS QDs/SiO2 composite films were applied to Si solar cells as luminescent down-shifting layers and the effect of QDs on the photoelectric conversion efficiency of photovoltaic modules was investigated. Under one sun illumination, the composite film containing an appropriate amount of CdSe/CdS/ZnS QDs effectively enhances the photoelectric conversion efficiency of the Si solar cell by spectral down-shifting as compared to the bare glass substrate, and the maximum achieves 16.14%.
Introduction
Crystalline silicon solar cells are still the leading technology for solar power conversion with high efficiency and large-scale production modules.1–4 However, the commercially produced photovoltaic (PV) modules usually show lower external quantum efficiency (EQE) at ultra-violet (UV) and blue regions compared to their EQE at longer visible wavelengths.5 Therefore, recently, researchers have paid much attention to how to improve the EQE at short wavelengths for enhancing the photoelectric conversion efficiency (η) of PV devices. The luminescent down-shifting (LDS), first proposed in 1979 by Hovel et al.,6 is considered as one of the effective strategies to increase solar cell efficiency.1,7–9 The principle of LDS is to convert UV/blue light with lower sensitivity to the solar cell to visible light with higher one, the higher short circuit current (Jsc) can be generated by this luminescent process because of the increase in the number of created electron–hole pairs per incident photon, and hence η of PV devices can be improved by LDS process.3Among the different down-shifting materials, rare-earth doped phosphors5,8,10–15 and organic fluorescent dyes16–18 were first investigated. However, the expected high efficiency in practice was not easy to reach due to the poor photostability under long-term UV light radiation and a limited absorption spectrum of organic dye molecules as well as extremely low absorption coefficient and a large particle size of phosphors. As an alternative, semiconductor quantum dots (QDs)1,2,7,9,19–25 have many advantages over rare-earth doped phosphors and organometallic complexes, such as a very broad absorption band, tunable emission wavelength by controlling their size and relatively good photostability.26–28 The potential for efficiency enhancement via QDs as a LDS layer has been recently reviewed by many authors.1,2,9,29 Cheng et al. employed CdS QDs-embedded silica film as a LDS layer on the front side of a crystalline Si solar cell to improve Jsc by maximum 4.0% but no η data were present.2 When CuInS2/ZnS core/shell QDs were used as the down-conversion material, the conversion efficiency of a Si-based solar cell showed a marked increase of 10.5% relative to the low efficiency (only 8%) of bare cell.29 Hodgson and co-workers9 reported an increase of 1.7% in conversion efficiency by applying CdSxSe1−x/ZnS core/shell QDs as a LDS layer to CdTe PV devices. Although recent results have indicated that modules with LDS layers containing QDs can offer efficiency improvements, the absolute η of cells is unsatisfactory because of the low photoluminescent quantum yield (PLQY) and small Stokes shift of the used QDs2,9,21,22,30 Therefore, the synthesis of high quality QDs is the key issue for improving efficiency of PV devices by QDs LDS layer.
We have previously reported one-step microwave-assisted synthesis of aqueous CdSe QDs. While these nanoparticles had large Stokes shift and were successfully applied to white light-emitting diodes (LEDs) to improve the color rendering, a low QY of 7.9% was unsatisfactory.26 To enhance the fluorescence and stability of the core particle, protective shells with a higher band gap are often grown around them.31,32 Based on this theory, high quality CdSe/CdS/ZnS core/shell/shell QDs were synthesized by coupling the microwave reactor to a fluorescence spectrometer via fiber optics. When combined with UV-visible light exposure during nucleation and after microwave-based QDs growth, CdSe/CdS/ZnS QDs with QY of 40% were obtained.33 However, this method used separate nucleation and growth steps for CdSe cores via oil bath and microwave, respectively, which makes the whole manipulations complex and limits its application in large-scale production. Therefore, a single step method will be optimum and is the pursuit of this study.
In this paper, water-dispersed CdSe/CdS/ZnS core/shell/shell QDs were synthesized for each component via a simple one-pot microwave irradiation (Fig. 1). The reaction time was reduced largely, and the whole experiment was completed after 30 min. As-prepared CdSe/CdS/ZnS QDs not only possess a high PLQY (25.4%) but also exhibit the excellent photostability and a large Stokes shift. When they were first embedded into SiO2 transparent matrix to form a composite film as a LDS layer for Si solar cell, the conversion efficiency of the cell shows a marked improvement of 5.2% in compared to the one with pure glass on the top.
 | ||
| Fig. 1 Schematic representation of preparation for CdSe/CdS/ZnS core/shell/shell QDs via a microwave route. | ||
Experimental
Chemicals
Cadmium chloride (CdCl2·2.5H2O, 99%), zinc chloride (ZnCl2, 99.5%), selenium powder (Se, 99.5%), 3-mercaptopropionic acid (3-MPA, 99%), sodium hydroxide (NaOH, 96%), sodium sulfite (Na2SO3, 95%), sodium sulfide (Na2S·9H2O, 99%), ethanol (C2H5OH, 99.7%), tetraethyl orthosilicate (TEOS, AR), 2-propanol (C3H8O, 99.7%), and Rhodamine 6G (99%) were purchased from Sinopharm Chemical Reagent Co., Ltd. Deionized water was used as a solvent. All reagents were used without further experimental purification.The preparation of precursors
Se precursor: 20 mg Se powder and 126 mg Na2SO3 were put into a special vessel containing 20 mL deionized water following a strong stirring. Then the vessel was heated to 100 °C in air for 20 min by microwave irradiation with a power of 100 W to obtain a clear Na2SeSO3 solution.Cd precursor: 57.1 mg CdCl2 was dissolved into 200 mL deionized water and 55 μL MPA was used as a surfactant. Then, 1 M NaOH was added to adjust the pH value of the reaction solution to 9.5.
Synthesis of thiol-capped CdSe core QDs
19 mL Cd precursor and 1 mL Se precursor were added into a microwave reaction vessel and heated to 100 °C for 30 seconds by microwave in the air. Then, it was quickly cooled down to 50 °C within 2 min by the high-pressure air. CdSe QDs with the emission wavelength at 572 nm was obtained. As-prepared CdSe QDs were precipitated with 2-propanol, collected by centrifuging, and re-dispersed in deionized water (1 mL) for further characterization and application.Synthesis of CdSe/CdS core/shell QDs
As-prepared CdSe core QDs were added to a mixture solution containing 1.25 mM CdCl2, 1.0 mM Na2S, and 6 mM MPA and the pH value of the solution was adjusted to 9.5. Then, the above mixture solution was placed in a microwave reaction vessel and reacted at 100 °C for 5 min. CdSe/CdS core/shell QDs were produced, precipitated with 2-propanol, collected by centrifuging, and re-dispersed in deionized water (1 mL) for further characterization and application.Synthesis of CdSe/CdS/ZnS core/shell/shell QDs
First, as-prepared CdSe/CdS QDs were added to a mixture solution containing 1.25 mM ZnCl2, 1 mM Na2S, and 6 mM MPA and the pH value of the solution was adjusted to 9.5. Second, the above mixture solution was loaded into a microwave reaction vessel and reacted at 70 °C for 5 min. Third, the resultant CdSe/CdS/ZnS core/shell/shell QDs were precipitated with excess 2-propanol, collected by centrifuging, and re-dissolved in deionized (1 mL) for further characterization and application.Preparation of QDs LDS layer for Si solar cells
In this paper, we used silica as a transparent matrix for encapsulating QDs. The silica sol was prepared through a sol–gel method as our previous work.2,10 As-prepared CdSe/CdS/ZnS QDs aqueous solution were added into the synthesized silica sol and uniformly dispersed by strong stirring. The resultant suspension solution containing QDs was deposited on the slide glass substrate by a dip-coating method with a pulling speed of 35 mm min−1 using a Czochralski machine. Before dip-coating, optical glass substrates (>90% transmittance in the wavelength range of 350–800 nm) were cleaned thoroughly in an ultrasonic bath. After dip-coating on the glass, the resulting CdSe/CdS/ZnS QDs/SiO2 composite thin films were tempered at 200 °C for 1 h in argon atmosphere. In this work, pure glass and pure silica films on glass are named as Sample 1, and Sample 2. The composite films with various CdSe/CdS/ZnS QDs concentrations from 0.2 mg L−1, 0.6 mg L−1, to 0.8 mg L−1 on glass were named as Sample 3, Sample 4, and Sample 5, respectively.Characterization
Absorption and transmission spectra of QDs and thin films were measured on a UV/vis spectrophotometer (Hitachi U-3900). Photoluminescence (PL) spectra were investigated using a fluorescence spectrophotometer (Horiba Jobin Yvon, FluoroMax-4) with a 150 W Xe lamp as an excitation source. PLQY was calculated by the following equation:where QY, D, A, and n are quantum yield, the optical density, the integrated area of PL spectrum, and the refractive index of QDs and dye, respectively. The optical density at the first absorption peak of both sample and dye were kept at 0.05–0.1 for avoiding reabsorption. In this paper, Rhodamine 6G dissolved in ethanol (PLQY = 95.0%, λex = 480 nm) was used as a standard dye. The morphology and microstructure of QDs were characterized by a JEOL-2100 transmission electron microscope (TEM). The photocurrent–voltage (J–V) measurement of solar cells was performed with a Keithley model 2440 Source Meter and a Newport solar simulator system (equipped with a 1 kW xenon arc lamp, Oriel) at one sun (AM 1.5 G, 100 mW cm−2). The active area of solar cell was 4 cm2.
Results and discussion
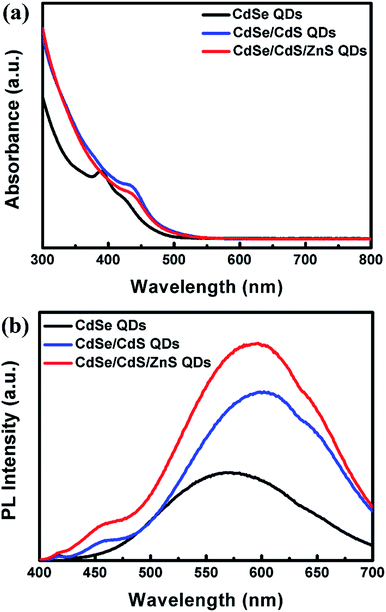
Fig. 2 displays the UV/vis absorption and PL spectra of CdSe core QDs, CdSe/CdS core/shell QDs, and CdSe/CdS/ZnS core/shell/shell QDs synthesized by microwave irradiation. It can be seen that CdSe core QDs show a sharp excitonic absorption band peaked at 390 nm and a broad yellow emission centered at 575 nm. A large Stokes shift of 8250 cm−1 avoids the self-absorption between QDs. After successfully growing a CdS shell around the host CdSe by microwave irradiation, about 50 and 25 nm redshifts for UV/vis absorption and PL spectra, respectively, could be observed. Simultaneously, PL intensity is enhanced by a large margin and the corresponding PLQY rises from 7.9% to 14.5%. An additional weak shoulder peaked at 460 nm from band-gap emission of host appears due to the enhancing luminescence. Further formation a ZnS shell on the CdSe/CdS QDs, the absorption and emission spectra of as-prepared CdSe/CdS/ZnS QDs present a slight redshift of 5 nm compared to CdSe/CdS QDs, moreover, PL intensity is superior to that of corresponding CdSe/CdS QDs since the ZnS shell effectively reduce the defects on the surface of the QDs in further. As a result, highly luminescent CdSe/CdS/ZnS QDs with a maximum PLQY of 25.4% are obtained by microwave irradiation. Red emission at 605 nm matches well with the spectral response for Si solar cells ranging from 400 to 1000 nm, so that as-prepared CdSe/CdS/ZnS QDs as a LDS material is expected to be more efficient for improving the efficiency of the cell. It is worth mentioning that the observed redshift in PL spectra is an indication for the formation of the intended CdSe/CdS/ZnS core/shell/shell structure rather than a Cd–Zn–Se–S alloy. The formation of alloyed QDs would lead to a blue-shift in both UV/vis absorption and PL spectra because of the larger band gap energy of alloyed QDs.34–36 Here, a CdS shell, whose band gap energy and lattice constant is between those of CdSe and ZnS, is introduced as a buffer layer between the CdSe core and the ZnS shell to avoid the serious strain generated by directly growing the ZnS shell on to the surface of CdSe QDs due to their large lattice mismatch (10.6%). This double shell structure allows a stepwise change of lattice spacing from the emitting CdSe core to the protecting ZnS shell, which reduces the strain within the QD.37,38 Moreover, charge carriers are effectively confined within the core region and separated from the surface due to the adequate offset of band gap energies (∼2 eV) between core and double-shell region. This reduces non-radiative surface defects and as a result leads to the high PLQY.34,39Good repeatability was prerequisites for QDs acquiring good applications in various fields. Therefore, the repeatability of preparing CdSe/CdS/ZnS QDs via microwave irradiation was investigated by repeating the experiment 9 times at the same conditions and their UV/vis absorption spectra are given in Fig. 3. All samples from S1 to S9 exhibit the same absorption band, which demonstrates the excellent repeatability of microwave-assisted route. That is to say, one-pot microwave method used in the present work is fully adaptable for mass production QDs only via repeating manipulation controlled by computer. Also, it can be easily employed to synthesize various other metal sulfide QDs.
 | ||
| Fig. 3 The UV/vis absorption spectra of CdSe/CdS/ZnS QDs prepared by microwave irradiation repeating 9 times at the same conditions. | ||
Fig. 4 shows transmission electron microscopy (TEM), high resolution TEM (HRTEM), and selected area electron diffraction (SAED) images of as-prepared CdSe, CdSe/CdS, and CdSe/CdS/ZnS QDs. Most of QDs exhibit nearly spherical morphology with a narrow size distribution. The average grain size is measured as 3.2 nm, 3.7 nm, and 3.9 nm for CdSe, CdSe/CdS, and CdSe/CdS/ZnS QDs, respectively, which corresponds well with the absorption spectra in Fig. 2. The increment in crystalline size results from the growth of the first CdS shell and the second ZnS shell and suggests an optimal thickness of 0.5 and 0.2 nm for the CdS and ZnS shell, respectively. HRTEM images of the core, core/shell, and core/shell/shell QDs show obvious lattice planes that extend across the entire particle with no evidence of an interface between the core and shell. Also, the SAED images of three kinds of QDs clearly give the same three diffraction rings of (111), (220), and (311) planes from inner to outer, respectively, which implies the identical crystal structure to that of bulk cubic zinc blende. The corresponding lattice parameter was calculated and is 0.6075 nm, 0.5965 nm, and 0.5894 nm for CdSe, CdSe/CdS, and CdSe/CdS/ZnS QDs, respectively. The lattice shrinkage is attributed to the decrease of ion radius from Cd2+ to Zn2+ and Se2− to S2−. All these microstructures further demonstrates the formation of core/shell/shell structure rather than alloyed structure and the shell growth does not disturb the crystalline form of the core.34
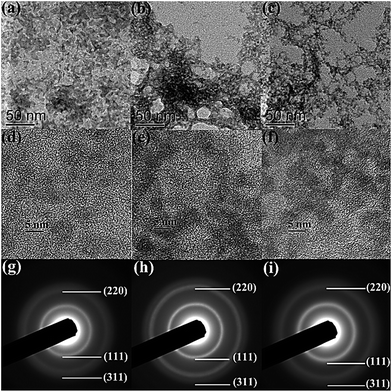 | ||
| Fig. 4 TEM (a–c), HRTEM (d–f), and SAED (g–i) images of CdSe, CdSe/CdS, and CdSe/CdS/ZnS QDs, respectively. | ||
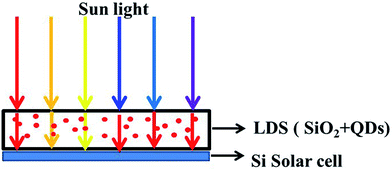
The schematic diagram of QDs/SiO2-based LDS layer applying to Si solar cell device is given in Fig. 5. It is well known that Si PV devices do not efficiently respond to photons of all wavelengths. The spectral response is significantly poorer for UV and blue light compared to longer visible wavelengths. Photons in the range of <400 nm interact strongly with the front layers of PV devices and are lost as heat in Si due to their high energy content. As-prepared CdSe/CdS/ZnS QDs were uniformly dispersed into SiO2 matrix to form a transparent composite thin film that was applied to the front of Si solar cell as a LDS layer.
Fig. 6 shows the UV/vis absorption and PL spectra as well as transmissivity curves of different composite thin films from Sample 1 to Sample 5. Compared with PL spectra of glass and pure SiO2 film, there is an obvious absorption and emission band covering from 340 to 500 nm and 550 to 750 nm, respectively, for QDs/SiO2 composite films, which is obviously due to the contribution from CdSe/CdS/ZnS QDs. As a result, the absorption and emission intensity gradually increases with QDs concentration. However, a decrease in transmissivity is accompanied when QDs concentration is too high and exceeds 0.6 mg L−1 (Sample 5), which finally has a negative impact on the conversion efficiency of the solar cell.40
 | ||
| Fig. 6 The UV/vis absorption (a) and PL (b) spectra as well as transmissivity (c) of different thin films from Sample 1 to Sample 5. | ||
The J–V curves of the Si solar cells containing different thin films on top are shown in Fig. 7. The short-circuit density (Jsc), open circuit potential (Voc), fill factor (FF), and conversion efficiency (η) of all the cells are listed in Table 1. It can be observed that the Jsc, FF, and η have obviously increased from 37.14 mA cm−2, 69.91%, and 15.34% for the blank substrate Si solar cell (Sample 1) to 38.11 mA cm−2, 71.16%, and 16.14% for the SiO2 composite thin film containing 0.6 mg L−1 CdSe/CdS/ZnS QDs (Sample 4). The high energy UV-blue lights with lower sensitivity to the solar cell are absorbed by CdSe/CdS/ZnS QDs and converted to red photons with higher sensitivity to the solar cell. These converted photons can be absorbed by Si and converted into useful electrical energy more efficiently. Another positive effect of this conversion is that the resulting red photons are penetrating deeper into Si than the unconverted high energy photons that are in general absorbed near the surface of the cell. This means that the red photons are absorbed nearer the p–n junction of the cell and generate electrons and holes that are efficiently collected under the effect of the electric field of the junction. Both of the above effects cause an enhancement of 5.2% for the conversion efficiency.23,29,41 However, the conversion efficiency decreases when the concentration of QDs in SiO2 film is further increased to 0.8 mg L−1 (Sample 5). This is because that high QDs concentration leads to poor film quality and as a result weakens the transmittance of the composite film shown in Fig. 6c.
| Sample | Jsc (mA cm−2) | Voc (V) | FF (%) | η (%) |
|---|---|---|---|---|
| 1 | 37.14 | 0.59 | 69.91 | 15.34 |
| 2 | 37.54 | 0.59 | 69.22 | 15.43 |
| 3 | 37.60 | 0.59 | 69.07 | 15.41 |
| 4 | 38.11 | 0.59 | 71.16 | 16.14 |
| 5 | 37.61 | 0.59 | 70.49 | 15.77 |
Conclusions
Water-dispersed CdSe/CdS/ZnS core/shell/shell QDs with high photoluminescence and a large Stokes shift were successfully synthesized by a facile microwave-assisted route. These QDs do not only possess a high PLQY but also excellent repeatability. The effect of CdSe/CdS/ZnS core/shell/shell QDs as LDS material on Si solar cell devices has been investigated by embedding them in transparent SiO2 films. The doping concentration was optimized. The conversion efficiency of the cell under one sun illumination is improved maximally 5.2% as compared to the one with pure glass as the concentration of the dots increases to 0.6 mg L−1.Acknowledgements
The authors gratefully acknowledge the financial support from National Natural Science Foundation of China (no. 51472087), Shanghai Municipal Natural Science Foundation (no. 13ZR1412500), Innovation Program of Shanghai Municipal Education Commission (no. 14ZZ050), the Fundamental Research Funds for the Central Universities (no. 78260022), the ECNU Reward for Excellent Doctoral Students in Academics (no. xrzz2014029), and the Large Instruments Open Foundation of East China Normal University.Notes and references
- S. Kalytchuk, S. Gupta, O. Zhovtiuk, A. Vaneski, S. V. Kershaw, H. Fu, Z. Fan, E. C. Kwok, C.-F. Wang and W. Y. Teoh, J. Phys. Chem. C, 2014, 118, 16393–16400 CAS.
- Z. Cheng, F. Su, L. Pan, M. Cao and Z. Sun, J. Alloys Compd., 2010, 494, L7–L10 CrossRef CAS PubMed.
- E. Klampaftis, D. Ross, K. R. McIntosh and B. S. Richards, Sol. Energy Mater. Sol. Cells, 2009, 93, 1182–1194 CrossRef CAS PubMed.
- B. Richards, Sol. Energy Mater. Sol. Cells, 2006, 90, 2329–2337 CrossRef CAS PubMed.
- T. Wang, B. Yu, Z. Hu, X. Wang, G. Zou and Q. Zhang, Opt. Mater., 2013, 35, 1118–1123 CrossRef CAS PubMed.
- H. J. Hovel, R. T. Hodgson and J. M. Woodall, Sol. Energy Mater., 1979, 2, 19–29 CrossRef CAS.
- J. Xu, S. Sun, Y. Cao, P. Lu, W. Li and K. Chen, Part. Part. Syst. Charact., 2014, 31, 459–464 CrossRef CAS.
- P. Liu, X. Zheng, X. Li, Z. Yao, X. Yu, X. Shi, B. Hou and Y. Xia, J. Mater. Chem. C, 2014, 2, 5769–5777 RSC.
- S. Hodgson, W. Brooks, A. Clayton, G. Kartopu, V. Barrioz and S. Irvine, Nano Energy, 2014, 4, 1–6 CrossRef CAS PubMed.
- C. B. Wang, T. T. Xuan, J. Q. Liu, H. L. Li and Z. Sun, Int. J. Appl. Ceram. Technol., 2014 DOI:10.1111/ijac.12281.
- N. Chander, A. Khan, P. Chandrasekhar, E. Thouti, S. K. Swami, V. Dutta and V. K. Komarala, Appl. Phys. Lett., 2014, 105, 033904 CrossRef PubMed.
- C. Huang, Y. Chen, W. Hung, T. Chen, K. Sun and W. L. Chang, Prog. Photovoltaics., 2013, 21, 1507–1513 CAS.
- Y. Xu, X. Zhang, S. Dai, B. Fan, H. Ma, J.-l. Adam, J. Ren and G. Chen, J. Phys. Chem. C, 2011, 115, 13056–13062 CAS.
- X. Y. Huang, X. H. Ji and Q. Y. Zhang, J. Am. Ceram. Soc., 2011, 94, 833–837 CrossRef CAS PubMed.
- D. Chen, Y. Wang and M. Hong, Nano Energy, 2012, 1, 73–90 CrossRef CAS PubMed.
- M. Buffa, S. Carturan, M. Debije, A. Quaranta and G. Maggioni, Sol. Energy Mater. Sol. Cells, 2012, 103, 114–118 CrossRef CAS PubMed.
- L. Danos, T. Parel, T. Markvart, V. Barrioz, W. Brooks and S. Irvine, Sol. Energy Mater. Sol. Cells, 2012, 98, 486–490 CrossRef CAS PubMed.
- E. Klampaftis and B. Richards, Prog. Photovoltaics, 2011, 19, 345–351 CAS.
- S. D. Hodgson, W. S. Brooks, A. J. Clayton, G. Kartopu, D. A. Lamb, V. Barrioz and S. J. Irvine, Prog. Photovoltaics, 2013 DOI:10.1002/pip.2408.
- S. D. Hodgson, W. S. Brooks, A. J. Clayton, G. Kartopu, V. Barrioz and S. J. Irvine, Nano Energy, 2013, 2, 21–27 CrossRef CAS PubMed.
- M. Dai Prè, I. Morrow, D. J. Martin, M. Mos, A. Del Negro, S. Padovani and A. Martucci, Mater. Chem. Phys., 2013, 139, 531–536 CrossRef PubMed.
- X. Pi, Q. Li, D. Li and D. Yang, Sol. Energy Mater. Sol. Cells, 2011, 95, 2941–2945 CrossRef CAS PubMed.
- S. M. Geyer, J. M. Scherer, N. Moloto, F. B. Jaworski and M. G. Bawendi, ACS Nano, 2011, 5, 5566–5571 CrossRef CAS PubMed.
- T. T. Xuan, S. Wang, X. Wang, J. Q. Liu, J. Y. Chen, H. L. Li, L. k. Pan and Z. Sun, Chem. Commun., 2013, 49, 9045–9047 RSC.
- H. Zhou, G. Zhou, J. Zhou, D. Xu, X. Zhang, P. Kong and Z. Yu, RSC Adv., 2014, 4, 42316–42325 RSC.
- T. T. Xuan, X. J. Wang, G. Zhu, H. L. Li, L. K. Pan and Z. Sun, J. Alloys Compd., 2013, 558, 105–108 CrossRef CAS PubMed.
- T. T. Xuan, X. J. Wang, J. Q. Liu, H. L. Li, L. K. Pan and Z. Sun, J. Mater. Chem. C, 2013, 1, 4550–4555 RSC.
- W. Zhang, H. Zhang, Y. Feng and X. Zhong, ACS Nano, 2012, 6, 11066–11073 CAS.
- S. Gardelis and A. G. Nassiopoulou, Appl. Phys. Lett., 2014, 104, 183902 CrossRef PubMed.
- A. Le Donne, S. K. Jana, S. Banerjee, S. Basu and S. Binetti, J. Appl. Phys., 2013, 113, 014903 CrossRef PubMed.
- M. A. Hines and P. Guyot-Sionnest, J. Phys. Chem., 1996, 100, 468–471 CrossRef CAS.
- L. Spanhel, M. Haase, H. Weller and A. Henglein, J. Am. Chem. Soc., 1987, 109, 5649–5655 CrossRef CAS.
- A. Zane, C. McCracken, D. A. Knight, W. J. Waldman and P. K. Dutta, J. Phys. Chem. C, 2014, 118, 22258–22267 CAS.
- Y. He, H. T. Lu, L. M. Sai, Y. Y. Su, M. Hu, C. H. Fan, W. Huang and L. H. Wang, Adv. Mater., 2008, 20, 3416–3421 CrossRef CAS.
- D. Pan, Q. Wang, S. Jiang, X. Ji and L. An, Adv. Mater., 2005, 17, 176–179 CrossRef.
- P. Yang, H.-S. Chen, S. Zhang, J. Zhao, Y. Du, Y. Miao, H. He and Y. Liu, RSC Adv., 2014, 4, 43800–43805 RSC.
- D. V. Talapin, I. Mekis, S. Götzinger, A. Kornowski, O. Benson and H. Weller, J. Phys. Chem. B, 2004, 108, 18826–18831 CrossRef CAS.
- J. Aguilera-Sigalat, S. Rocton, J. F. Sanchez-Royo, R. E. Galian and J. Perez-Prieto, RSC Adv., 2012, 2, 1632–1638 RSC.
- A. Aharoni, T. Mokari, I. Popov and U. Banin, J. Am. Chem. Soc., 2006, 128, 257–264 CrossRef CAS PubMed.
- J. Liu, K. Wang, W. Zheng, W. Huang, C. H. Li and X. Z. You, Prog. Photovoltaics, 2013, 21, 668–675 CAS.
- R. Rothemund, S. Kreuzer, T. Umundum, G. Meinhardt, T. Fromherz and W. Jantsch, Energy Procedia, 2011, 10, 83–87 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |