Metal-free synthesis of imidazopyridine from nitroalkene and 2-aminopyridine in the presence of a catalytic amount of iodine and aqueous hydrogen peroxide†
Abstract
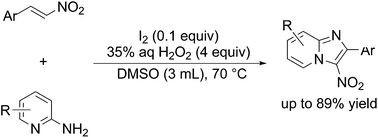
We have developed a metal-free synthetic method for 3-nitroimidazo[1,2-a]pyridines from nitroalkenes and 2-aminopyridines using catalytic amounts of iodine and aqueous hydrogen peroxide as a terminal oxidant.

 Please wait while we load your content...
Please wait while we load your content...