Enhanced CO2 transport properties of membranes by embedding nano-porous zeolite particles into Matrimid®5218 matrix
Abstract
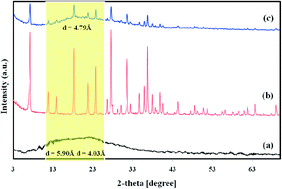
A novel mixed matrix membrane (MMM) was fabricated by incorporating micro-sized nano-porous sodium zeolite-Y (NaY zeolite) into Matrimid®5218 matrix. The filler and the prepared membranes were characterized by X-ray diffraction (XRD), Fourier transform infrared-attenuated total reflectance (FTIR-ATR), scanning electron microscopy (SEM), and thermal gravimetric and derivative thermal gravimetric (TG/DTG) analyses. The effects of filler content (0–20 wt%), feed pressure (2–12 bar), operating temperature (35–75 °C) and mixed feed gas on CO2/CH4 transport properties of Matrimid/NaY were investigated. The results revealed that the Matrimid/NaY (15 wt%) displayed a CO2 permeability of 17.52 Barrer, more than two-fold increase with respect to the NaY-free counterpart. The corresponding CO2/CH4 selectivity was increased from 36.3 for Matrimid to 43.3 for Matrimid/NaY (15 wt%), (about 20%). The CO2 permselectivities of MMMs were greater than that of the Matrimid over the entire pressure range. As the operating temperature increased from 35 to 75 °C, CH4 permeability increased about 175% and 215% for Matrimid and Matrimid/NaY (15 wt%), respectively. While the CO2 permeability enhanced about 78% and 98%. The corresponding decreases in the CO2/CH4 selectivities were 35.27% and 37.14%, respectively. Moreover, the mixed gas experiment results indicated that CO2 permeability and CO2/CH4 selectivity for all membranes were lower than those of pure gas experiments, but with less severity for MMMs. The best CO2-selective membrane, Matrimid/NaY (15 wt%), represented the CO2 permeability of 15.19 Barrer with CO2/CH4 selectivity of 39.5 for a 10/90 vol% mixture of CO2 and CH4.

 Please wait while we load your content...
Please wait while we load your content...