A DFT study on an alkali atom doped decahedral silver nanocluster for potential application in opto-electronics and catalysis
Shaikat Debnatha,
Suhana Mohd Said*a,
Muhammad Faris Roslana,
Mohd Faizul Mohd Sabrib and
Bui Duc Longb
aDepartment of Electrical Engineering, Faculty of Engineering, University of Malaya, Kuala Lumpur 50603, Malaysia. E-mail: smsaid@um.edu.my
bDepartment of Mechanical Engineering, Faculty of Engineering, University of Malaya, Kuala Lumpur 50603, Malaysia
First published on 22nd December 2014
Abstract
A systematic study of the structural, electronic and optical properties of the decahedral bimetallic Ag12X cluster is presented in the framework of density functional theory (DFT), where one atom of an alkali metal (X = Li, Na, K, Rb, Cs, Fr) is added, replacing a Ag atom in the decahedral Ag13 cluster in core (c-doped), vertex (v-doped) and surface (s-doped) positions. Geometrical optimization of the clusters indicated that Li and Na doped clusters exhibited the highest stability. The binding energy (BE), vertical ionization potential (VIP), vertical electron affinity (VEA) and HOMO–LUMO gaps were calculated to compare the electronic stability and chemical inertness of the doped clusters. In addition, the VIP and VEA values of the doped clusters revealed that the doped clusters exhibited more electronic and chemical reactivity than the undoped Ag13. Through optical spectra analysis, it is revealed that Ag12Na and Ag12Li clusters exhibited higher oscillation strength, whilst the s-doped Ag12Li exhibited 3 times higher oscillation strength with respect to undoped Ag13. In addition, a partial density of states (PDOS) calculation indicated that the red or blue shifting of the d-electrons are potentially responsible for this red and blue shifting of the optical peaks of the doped Ag12X clusters. Finally, these Ag12X clusters have promising electronic and optical properties; in particular, the Ag12Li dimer is a highly stable cluster with excellent optical absorption spectra. Thus, a neutral Ag12Li cluster might find good application in opto-electronics and its anion might be highly reactive and thus, can be a very good potential candidate for catalysis.
1. Introduction
Over the past few decades, the progress in nanotechnology has impacted every aspect of science and technology. Crucial to developments in nanotechnology is the design and synthesis of nanoparticles. Silver nanoparticles have been a subject of intense interest, given their distinct physical and chemical properties. This special feature of Ag nanoparticles has provided a versatile platform for a range of diverse applications ranging from photovoltaic1 to biological2 and chemical sensors3 and catalysis.4 Amongst the shapes of the silver nanoparticles used to date, decahedron shaped particles are of special interest. In the morphology of the metal nanoparticles, pentagonal cyclic twinning (PT) is considered as a special feature.5 In the last few decades many reports have been found regarding symmetric metal nanoparticles with PT such as rods, cubes, plates, icosahedra, decahedral, truncated octahedral and octahedral geometries.6–10 From the aforementioned shapes, the decahedron (pentagonal bi-pyramids, Johnson solid J13) with D5h symmetry is the most compact PT structure, hence emphasizing the importance of decahedron shaped Ag nanoparticles.10The optical properties of Ag nanoparticles are of special interest, due to the fact that Ag nanoparticles support surface plasmon resonance (SPR). SPR is defined as the collective oscillation of electrons in a solid or liquid stimulated by incident light and the resonance condition for SPR is established when the frequency of light photons matches the natural frequency of surface electrons oscillating against the restoring force of positive nuclei.11 The plasmonic behaviors of the silver nanoparticles are size and shape dependent. For example, the decahedron shaped Ag nano-particles exhibit remarkable optical absorption properties,8,10 which has made decahedral silver nanoparticle a popular commercial product such as SCIVENTION's 35–120 nm sized decahedral silver, which are used in opto-electronic, catalytic and biological applications. On the other hand, alkali metals are the elemental free-electron metals, which have been identified as excellent potential for plasmonic nanometals. M. G. Blaber et al. have reported12,13 that sodium and potassium have the highest absorption efficiency (Qabs), along with very low inter-band transition losses at optical frequencies, compared to the nanometals that have been tested to date. In fact, these transition losses are comparable or even better than that of silver along with their property of exhibiting the strongest free-electron-like-behavior, which result in very prominent SPR in visible-UV range.14 Thus, elemental free electron metals (alkali metals) addition into the decahedral Ag nanocluster may be considered for potential improvement of the optical absorption of the silver nanoparticles, which will be an interesting study to explore. Moreover, both of the alkali metals and silver have valence s-electrons; hence rendering the bimetallic system simple. Furthermore, the large difference in electronegativity between Ag and alkali metals makes it favorable for mixing. On the other hand, the large gap for the cohesive energies and atomic radii between the silver and alkali metals favor the core–shell segregation, which is another key reason for selecting the Ag–alkali metal bimetallic clusters as the model for computation.15
Thus, in our current work, we have selected the 13-atoms decahedral shaped Ag clusters to be doped by the alkali metals (X = Li, Na, K, Rb, Cs, Fr) in core, surface and vertex positions, which will be referred to c-doped, s-doped and v-doped Ag12X bimetallic clusters respectively. Recent DFT calculations16,17 have shown that the most stable structure is an entirely deformed structure with C1 symmetry which actually contradicts the findings of previous empirical studies that suggested the icosahedron structure as the lowest energy isomer.18 Thus, decahedral shaped Ag in 13-atoms level is not actually the global minima. Decahedral silver in the size range of 35–120 nm has been established as a commercially available product for use in various opto-electronic, catalytic and biological applications. Thus this work explores the potential improvement of the Ag clusters properties through doping of alkali atoms into silver. Therefore, as a first investigation, our 13-atom decahedral Ag12X bimetallic clusters can be treated as the quantum models for the Ag–X bimetallic clusters.6 Whilst the electronic and optical properties of any material's nanoparticle changes with the change of the size, this quantum model will provide an indication of the trend of changes in electronic and optical properties as the result of doping alkali metals into decahedral silver. Several reports on theoretical study and photoionization spectroscopy of metal dimers including Ag–Li,19,20 Ag–Na,21 Ag–K,22 Ag–Rb15 have been reported but we believe that this is the first DFT study with regards to the structural, electronic and optical properties of Ag–X bimetallic clusters in 13-atoms level.
To study the optical spectra of nanoparticles and bimetallic nanoclusters, several theories have been implemented such as the Mie theory,23 the discrete dipole approximation (DDA)24 and the electromagnetic finite difference time domain (FDTD).25 At present, time dependent density functional theory (TDDFT)26 is gaining popularity as the simulation tool of choice to investigate the optical properties of noble metal clusters. This is because the quantum mechanical TDDFT has been found to be satisfactory in unfolding small (i.e. less than 20 atoms) nanoclusters with detailed explanation of the electron extinction, which is justified by several reports27–29 which has demonstrated that TDDFT calculations are satisfactory in explaining the relationships between SPR and the structure and size of pure noble metal nanoclusters of decahedral, octahedral and icosahedral structures.
In this paper, a systematic investigation of electronic and optical properties of doped neutral clusters of Ag12X is explored. For this purpose, a single silver atom has been replaced by an alkali metal atom (X = Li, Na, K, Rb, Cs, Fr) to form the decahedral Ag12X bimetallic clusters. With the geometrically optimized structures, DFT based calculations of binding energy, HOMO–LUMO gap; vertical ionization potential (VIP) and vertical electron affinity (VEA) were carried out to characterize the stability and chemical inertness of the doped Ag12X bimetallic clusters. TDDFT calculation has been carried out to predict the optical spectra of the clusters, which is then followed by the partial density of states (PDOS) calculations.
In the next section, the computational method will be described briefly. Structural and electronic properties will be presented and discussed in Section 3, which is then followed by optical spectrum and PDOS of the clusters.
2. Computational method
In this study, the investigation of geometrical and energetic stabilities and optical properties of decahedral Ag13 with D5h symmetry for the doped Ag12X bimetallic structures were investigated using the density functional spin-polarized calculations using DMol3 Accelrys Inc. code.30,31 All geometrical structures of the neutral clusters are optimized with imposing symmetry. Calculations have been performed using the Perdew–Burke–Ernzerhof (PBE) exchange–correlation functional.32 The Kohn–Sham equation was expanded in a double numeric quality basis set (DNP) with polarization functions. To consider the relativistic effect, the DFT semi-core pseudo-potentials33 are used for the treatment of the core electrons of the doped clusters. The orbital cut off range and Fermi smearing were selected as 5.0 Å and 0.001 Ha respectively. The self-consistent-field (SCF) procedures were performed with the aim of obtaining well converged geometrical and electronic structures with a convergence criterion of 10−6 a.u. The energy, maximum force and maximum displacement convergence were set as 10−6 Ha, 0.002 Ha Å−1 and 0.005 Å respectively. The cationic and anionic structures are also optimized with same functionals and basis set for computing the values of Ionization Potential (IP) and Electron Affinity (EA). The ALDA kernal exchange co-relation method was employed with TDDFT for calculating the optical excitation spectra.34 In addition, Gaussian broadening is applied to the eigen values here to get the optical spectra of the clusters.3. Results and discussion
3.1. Geometric structures and energetic stability
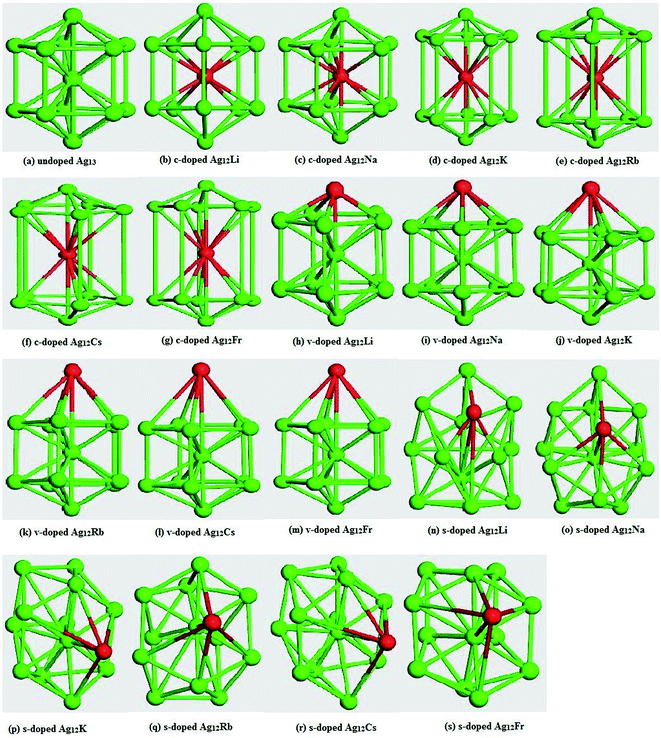
In order to check the ground state structures, electronic and optical properties of the alkali atoms doped Ag12X clusters, one silver atom is replaced in core, surface and vertex of a 13 atoms Ag13 decahedral structure has been substituted by an alkali atom to form c-doped, s-doped and v-doped Ag12X bimetallic clusters respectively. The ground state of all clusters were determined by checking their total energy and binding energy with different spin multiplicity from singlet to octet and it was found that for Ag13 and all the doped clusters, the spin-1 (one) offers the most stable structures, which also confirms the findings of the previous reports.27–29 All the c-doped and s-doped Ag12X neutral and charged clusters are geometrically optimized with imposing symmetry by nonlocal functional Generalized Gradient Approximation (GGA) with PBE as exchange function and DNP as basis set. Here, it worth mentioning that decahedral in 13 atoms level is not a global minimum. Given that the objective of this work is to predict the trend of change in electronic and optical properties in decahedral silver as the result of alkali atoms doping, all the calculations were carried out by imposing symmetry constrains so that the doped Ag12X clusters maintain the similar or, at least adjacent symmetry of parent Ag13 cluster of D5h symmetry.Fig. 1 shows the optimized structures of the doped Ag12X clusters. Along with center doping, surface doping has been done is two positions; one in the vertex and the other one in the shell, which will be calling v-doped and s-doped clusters from now on. Now, from the Fig. 1, it is evident that all the c-doped clusters maintain the same D5h symmetry of the parent Ag13, whereas the v-doped and s-doped clusters reform to C5v and Cs symmetry respectively. To this end, examination of the geometric degree of deviation of the doped bimetallic clusters compared to parent Ag13 has been a focus of investigation and towards that end the calculation for the average Ag–X bond lengths of the entire Ag12X clusters and compiled those data in Table 1.
| c-doped clusters D5h symmetry | Average bond length Å | v-doped clusters C5v symmetry | Average bond length Å | s-doped clusters Cs symmetry | Average bond length Å |
|---|---|---|---|---|---|
| Ag–Ag | 2.91 | Ag–Ag | 2.90 | Ag–Ag | 2.85 |
| Ag–Li | 3.07 | Ag–Li | 3.49 | Ag–Li | 2.78 |
| Ag–Na | 3.18 | Ag–Na | 3.27 | Ag–Na | 3.15 |
| Ag–K | 3.29 | Ag–K | 3.82 | Ag–K | 3.51 |
| Ag–Rb | 3.71 | Ag–Rb | 3.94 | Ag–Rb | 3.69 |
| Ag–Cs | 4.12 | Ag–Cs | 4.14 | Ag–Cs | 3.99 |
| Ag–Fr | 4.17 | Ag–Fr | 4.18 | Ag–Fr | 4.01 |
Now, from the data of Table 1, it can be clearly seen that Ag12Li and Ag12Na clusters exhibit the minimum geometrical deviation, whereas the maximum deformation is observed from Ag12Cs and Ag12Fr clusters compared to undoped Ag13. Hence, it can be said that the Li and Na doped clusters are supposed to be more stable with respect to Cs and Fr doped clusters. This deforming trend can be easily explained considering the fact that the atomic radii of the doped alkali atoms increases from Li (1.52 Å) to Fr (2.82 Å), while it maintains an incremental trend (Na: 1.54 Å, K: 2.27 Å, Rb: 2.48 Å, Cs: 2.65 Å) down the periodic table group. Besides, all these atomic radii are larger than the atomic radii of Ag (1.44 Å).35 Thus, when doping similar sized atoms (for example Li, Na and) into Ag13 produces the minimum geometrical deviation with respect to the parent Ag13. Similarly, adding larger atoms results with higher geometrical deformation. Now, with the results of structural change as the outcome of doping, it is clearly understood that Li, Na and K doping are more favorable with respect to Rb, Cs and Fr. Moreover, being the bottom three elements of group-IA, it is well known that Rb, Cs and Fr are very reactive elements. Thus, doping with these three elements to reform Ag–alkali metals bimetallic nanoalloy are not practically feasible option. Therefore, in our next section, where will discuss the electronic and optical properties of the Ag–X clusters, our doped elements will be limited to Li, Na and K only, and accordingly X = Li, Na, K.
3.2. Electronic properties
In this paper, the focus is to evaluate the change in optical properties as a result of doping of alkali atoms into decahedral silver. But before checking the optical properties, it is important to check the electronic and chemical stability of the doped clusters in order to ensure it long term stability in targeted applications. Moreover, this calculation will allow us to check the potential of bimetallic clusters for possible application in catalysis. Therefore, after discussing the structural motifs of the doped clusters in the previous section, analysis is then focused on the electronic properties of the clusters and Tables 2 and 3 have listed the binding energy (BE), vertical ionization potential (VIP), vertical electron affinity (VEA) and HOMO–LUMO gap of the entire c-doped, v-doped and s-doped Ag12X clusters. In order to check the stability of the clusters, the average binding energy per atom is calculated in the following way, where EAg12X, EAg12 and EX refers to the energy of the Ag12X, Ag12 and X clusters accordingly and X = Li, Na, K.| Binding energy = EAg12X − [EAg12 + EX] |
| Cluster | Symmetry | Spin | BE (eV) | VIP (eV) | VEA (eV) | HOMO–LUMO gap (eV) |
|---|---|---|---|---|---|---|
| Ag13 | D5h | 1 | 19.19 | 5.61 | 2.08 | 0.22 |
| Ag12Li | D5h | 1 | 19.79 | 5.71 | 2.20 | 0.23 |
| Ag12Na | D5h | 1 | 18.71 | 5.75 | 2.16 | 0.19 |
| Ag12K | D5h | 1 | 17.29 | 5.73 | 2.79 | 0.17 |
| Cluster | Symmetry | Spin | BE (eV) | VIP (eV) | VEA (eV) | HOMO–LUMO gap (eV) |
|---|---|---|---|---|---|---|
| Ag12Li | C5v | 1 | 19.73 | 5.35 | 1.76 | 0.21 |
| Cs | 1 | 20.06 | 6.96 | 1.49 | 0.20 | |
| Ag12Na | C5v | 1 | 19.00 | 5.35 | 1.76 | 0.21 |
| Cs | 1 | 19.12 | 5.51 | 1.78 | 0.19 | |
| Ag12K | C5v | 1 | 18.20 | 5.32 | 1.41 | 0.16 |
| Cs | 1 | 19.08 | 5.57 | 1.32 | 0.17 |
Referring to Table 2, it can be said through the DFT calculation; the binding energy of decahedral Ag13 is predicted to be 19.19 eV, which is similar to the previously reported result from Yi Rao et al.29 Referring to the data of Tables 2 and 3, we can clearly find that amongst all the doped structures only Ag12Li possess higher BE than the corresponding value of Ag13; hence the Li doped Ag12Li neutral clusters are expected to be more stable than the undoped Ag13 clusters. In particular, the s-doped Ag12Li with Cs symmetry exhibits the highest BE amongst all the clusters considered in this paper. We also observe that the surface or, vertex doped Ag12X clusters displays higher BE compared to c-doped Ag12X compounds, signifying that the favored structure is c-doped Ag12Li and the entire surface doped Ag12X structures. The reason for this observation is as follows: the bulk interatomic distances for Ag, Li, Na, K are 2.89, 3.023, 3.659 and 4.525 Angstroms, respectively.36 Thus, the strain for c-doped Ag12X1 increases in the order X = Li, Na, K and is larger for the bigger atoms. Therefore, they prefer the surface doping site. However interestingly, c-doped Ag12Li has higher binding energy than Ag13.
Next, three more key indicators which are crucial for the evaluation of the chemical stability of small clusters are ionization potential (IP), electron affinity (EA) and HOMO–LUMO gap. Now, we know that the higher value of IP specifies a deeper HOMO energy level, signifying that the structures with large value of IP are expected to be chemically more stable or, exhibiting lesser chemical reactivity. On the contrary, a higher value of EA indicates a more solid binding between the cluster and an electron. Here it worth mentioning that, while calculating the IP and EA, it was assumed that the lowest-energy structure for a charged cluster is the same as their neutral cluster and the calculated IP and EA are actually vertical IP (VIP) and vertical EA (VEA).
From the range of c-doped, v-doped and s-doped Ag12X structures under study in Tables 2 and 3, undoped Ag13 exhibits 5.61 eV as VIP, which resembles the previous DFT study of Yi Rao et al.29 Amongst all the doped clusters, s-doped Ag12Li with Cs symmetry are found to have the largest value of IP (6.97 eV). It implies that Ag13 and other all other doped clusters lose electron more easily than s-doped (Cs symmetry) Ag12Li. Moreover, almost all the Ag12X clusters actually exhibit comparatively higher IP values with respect to undoped Ag13, which is due to the fact that the large gap of electronegativity between Ag and alkali metals have produced some kind of ionic bonding in the doped cluster, which results with higher IP.
On the other hand, the entire c-doped clusters exhibit higher VEA values than Ag13, whereas all the s-doped and v-doped clusters show lower VEA values with respect to Ag13. It suggests that it will take more energy to attach an electron into the c-doped clusters with respect to undoped or surface doped silver clusters. The probable reason behind these higher EA values can be that, when alkali atoms are doped in the center, the Ag atoms in the shell are pulled apart. This phenomenon creates large geometrical change in the cluster, which eventually result into higher values of VEA. On contrary, the low VEA values of the surface doped clusters can be easily explained by comparing the electronegativity between the Ag atoms and the doped atoms. It is a well-known fact that the group IA metals are highly electropositive. Thus, doping a high electropositive atom such as Li, Na or, K in the surface will enable the cluster to possess a higher affinity for electrons compared to the undoped Ag13 cluster.
Apart from the BE, VIP and VEA, the energy gap between the HOMO and LUMO is considered as an important quantity for evaluating the electronic stability of clusters. In fact, a cluster with high HOMO–LUMO gap is generally considered as a cluster with high kinetic stability and low chemical reactivity and vice versa. This is due to the fact that energetically it is not favorable to add electrons to a high lying LUMO and to extract electronics from a low lying HOMO, making it difficult to form the activated complex of any potential reaction.37,38 Now, from the data of Tables 2 and 3 it is revealed that GGA functionals predicted a value of 0.22 eV as the HOMO–LUMO gap of Ag13 clusters, which confirms the previous report.29 Amongst the doped clusters only c-doped Ag12Li possesses higher HOMO–LUMO gap with respect to Ag13; hence the aforementioned doped cluster is expected to be more stable and chemically inert than the undoped silver cluster. Also, it has been reported that neutrals clusters with high HOMO–LUMO gaps are expected to be very active as anions because of their lower values of VEA.39 From the results presented in Tables 2 and 3, it is clear that neutral doped clusters (especially Ag12Li) possess high BE and HOMO–LUMO gaps along with low VEA. Therefore, anions of these clusters are expected to be highly reactive compared to the corresponding of undoped Ag13, hence can be good potential candidates for catalysis.
3.3. Optical properties
After discussing the structural and electronic properties of the doped clusters, the attention is now towards the key motivation of this paper, which is the optical spectrum of the doped clusters. Here, TDDFT with ALDA kernel exchange–correlation terms in singlet state is used to calculate the optical spectra of the doped clusters. Fig. 2a shows the optical spectra of Ag13, where it is clearly seen that the silver cluster has its optical spectrum range in between 330 to 400 nm while its peak is found at 360 nm (3.44 eV) wavelength, which is an excellent match to the previously reported experimental spectra of Ag13.40,27 Vladimir Kitaev10 et al. has reported the experimental optical absorption spectra of the 35–123 nm sized decahedral silver nanoparticles in the range of 455–570 nm wavelength. However, it is well known that the optical peaks of the nanoparticles are red-shifted with the increment of the particle size.16,41 Thus absorption spectra of our investigated 13-atom silver cluster, which is significantly smaller with respect to those 35–123 nm sized particles, is consistent with the anticipation of a blue shift for smaller particles.Referring to the optical spectra of the doped clusters of Fig. 2b–d, the effect of doping in the optical properties can be described as follows. All the c-doped Ag12X clusters optical peak are red-shifted with respect to undoped Ag13, whilst c-doped Ag12Na exhibits excellent optical oscillation strength with its peak in the range of 370–390 nm wavelength. On the other hand, the entire v-doped clusters display wider absorption spectra ranged 300–600 nm compared to the parent Ag13. Moreover, the optical peak of v-doped Ag12Li and Ag12Na dimers are slightly blue-shifted, whereas a red-shifted peak was observed from Ag12K with respect to parent Ag13. The third group of doped clusters i.e. the s-doped clusters exhibit very interesting optical spectra. Amongst these clusters, the optical peak of Na-doped clusters is red-shifted, whilst the Li and K doped clusters show their optical peak exactly in the same wavelength of silver. But interestingly, the Li doped cluster exhibit remarkable absorption strength, which is almost 3 times higher than the undoped Ag13 dimer. On the contrary, it is reported that the increment of cluster size in a pure metallic nanoparticle system results to red-shift in optical bands, whereas blue-shift occurs as result of decrement of cluster size.16,41 Thus, it links up the relation between the optical spectra and the geometrical structures of the bimetallic clusters.
With reference to the optical spectra of the doped clusters, let's move our focus towards the electronic evolution of the spectra of the doped clusters. Here, with the presence of alkali atoms these bimetallic Ag12X clusters in the excited states lead to a quasi-continuum spectrum. In particular, for the case of Ag13, the electronic transitions are happening as a result of excitations from the s and d orbitals. These s and d orbitals are well localized in surface silver atoms, along with its evolution as hybrid s–p orbitals in the wide areas of outer region where several atoms are involved with the system.6 On the contrary, the absorption spectra of alkali metal clusters are explained as collective oscillation of s valence electrons and the shift of absorption energies of very small particles, compared to the larger ones explained by a spill out (extension of the electronic wave functions out of the “classical volume” of the cluster) of these s-electrons.16 Thus, when alkali metal is doped into the noble metal cluster, a systematic investigation of the excitations in molecular level is quite complicated. To this end, for investigating the absorption spectrum of the clusters we have presented the partial density of states (PDOS) calculation in Fig. 3, where the PDOS of Ag13, c-doped, s-doped and v-doped Ag12Li are presented.
 | ||
| Fig. 3 (a): PDOS of Ag13. (b): PDOS of c-doped Ag12X clusters. (c): PDOS of v-doped Ag12X clusters. (d): PDOS of s-doped Ag12X clusters. | ||
Now, from the PDOS of Fig. 3(a–d), it is clearly observed that the actions of d orbitals in Ag13 are more dominant in the in the energy range of −2 to −5.5 eV, with its peak at −3.15 eV. For the cases of c-doped, v-doped and s-doped Ag12Li, we find that the d electrons still exhibit the stronger role, but their peaks are red-shifted to −3.9 eV, −3.75 eV and −3.55 eV respectively, which indicates that d electrons are transferred to higher energy level for the doped clusters. In actual fact the doping of Li into the Ag nanoparticles results an increase in the occupation of empty s and p bands of the Ag13 cluster, which eventually causes the d electrons transferred to higher energy level. Thus, this transfer of d electrons potentially well-explain the red-shifting or, blue-shifting of optical spectra of the doped Ag12X clusters.
Overall, it can be concluded that the c-doped and v-doped Ag12Na and s-doped Ag12Li are the most favorable clusters if the higher oscillation strength is the criteria, whilst the entire c-doped and v-doped clusters exhibit wider ranged absorption spectra with respect to undoped Ag13.
4. Conclusion
In this paper, the ground state structures, electronic and optical properties of the doped and undoped silver clusters have been calculated in the framework of density functional theory (DFT and TDDFT). To investigate the doped cluster, one silver atom is replaced from the core, surface and vertex of a 13 atom Ag13 decahedral structure has been substituted by an alkali atom to form c-doped, s-doped and v-doped Ag12X bimetallic clusters respectively. Amongst the doped clusters, Li, Na and K doped clusters result least distortion from undoped Ag13 cluster; hence imply the best stability. To analyze the electronic and chemical stability of the doped clusters, the calculations of BE, VIP, VEA and HOMO–LUMO gaps of the Ag12X clusters were performed. It is observed that the Ag12Li clusters have higher BE than the parent Ag13 cluster; hence Li doped cluster is the more favored amongst all the doped clusters considered. All the doped clusters have exhibited high values of VIP; hence the doped clusters are expected not to lose electrons easily. Moreover, we observe higher VEA values for the c-doped clusters compared to Ag13 and the surface doped clusters; implying that the s-doped clusters hang around with electrons more easily with respect to c-doped Ag12X clusters. Regarding the HOMO–LUMO gap, all the doped clusters showed high values; hence anions of the doped clusters are expected to be very reactive.The optical properties of the Ag13 and Ag12X are calculated in the framework of TDDFT with ALDA-kernal as the exchange–correlation function and all the doped clusters actually exhibit remarkable optical absorption spectra. The c-doped and v-doped Ag12Na and s-doped Ag12Li have exhibited excellent oscillation strength, while the peak value of the s-doped Ag12Li is found 3 (three) times higher than the undoped silver cluster. In addition, the entire c-doped and v-doped clusters have exhibited wider ranged absorption spectra with respect to undoped Ag13. Thus, all of these clusters can be excellent potential in the applications of opto-electronics.
Finally, our study has concluded that the single-atom alkali metal doped Ag12X1 bimetallic nanoclusters have interesting properties regarding optical absorption spectrum and electronics. In particular, Li doped clusters highly stable with excellent optical absorption spectra. Therefore, it is expected that neutral Ag12Li clusters might find good application in opto-electronics and its anion (Ag12Li−) might be highly reactive and can be a very good potential candidate for catalysis.
Acknowledgements
The authors wish to acknowledge the University of Malaya–Ministry of Higher Education Grant UM.C/625/1/HIR/MOHE/ENG/29, University of Malaya Research Grant (UMRG) RP014C-13AET and Malaysian Ministry of Science and Technology's Science Fund (06/01/03/SF0831) for the financial support. Deepest thanks also go to Professor René Fournier, from York University, Canada and Dr Abhijit Chatterjee, from Accelrys K. K.; Japan Office, for the technical discussions and acknowledgment also to Prof. Dr Nasrudin Bin Abd Rahim and Universiti Malaya Power Energy Dedicated Advanced Centre (UMPEDAC) for the constructive support during the course of this work.References
- C. Photiphitak, P. Rakkwamsuk, P. Muthitamongkol, C. Sae-Kung and C. Thanachayanont, Int. J. Photoenergy, 2011, 2011, 258635 CrossRef.
- D. Singh, V. Rathod, S. Ninganagouda, J. Hiremath, A. K. Singh and J. Mathew, Bioinorg. Chem. Appl., 2014, 2014, 408021 Search PubMed.
- A. K. Yu, A. A. Kudrinskiy, A. Y. Olenin and G. V. Lisichkin, Russ. Chem. Rev., 2008, 77, 233 CrossRef PubMed.
- M. H. Rashid and T. K. Mandal, J. Phys. Chem. C, 2007, 111, 16750 CAS.
- C. L. Cleveland and U. Landman, J. Chem. Phys., 1991, 94, 7376 CrossRef CAS PubMed.
- M. Harb, F. Rabilloud and D. Simon, Phys. Chem. Chem. Phys., 2010, 12, 4246 RSC.
- K. Kwon, K. Y. Lee, Y. W. Lee, M. Kim, J. Heo, S. J. Ahn and S. W. Han, J. Phys. Chem. C, 2006, 111, 1161 CrossRef.
- M. Liu and P. Guyot-Sionnest, J. Phys. Chem. B, 2005, 109, 22192 CrossRef CAS PubMed.
- I. Pastoriza-Santos, A. Sánchez-Iglesias, F. J. Garcíade Abajo and L. M. Liz-Marzán, Adv. Funct. Mater., 2007, 17, 1443 CrossRef CAS.
- B. Pietrobon and V. Kitaev, Chem. Mater., 2008, 20, 5186 CrossRef CAS.
- S. Zeng, K.-T. Yong, I. Roy, X.-Q. Dinh, X. Yu and F. Luan, Plasmonics, 2011, 6, 491 CrossRef CAS.
- M. G. Blaber, M. D. Arnold and M. J. Ford, J. Phys. Chem. C, 2009, 113, 3041 CAS.
- M. G. Blaber, M. D. Arnold and M. J. Ford, J. Phys.: Condens. Matter, 2010, 22, 143201 CrossRef CAS PubMed.
- P. R. West, S. Ishii, G. V. Naik, N. K. Emani, V. M. Shalaev and A. Boltasseva, Laser Photonics Rev., 2010, 4, 795 CrossRef CAS.
- R. Fournier, S. Zamiruddin and M. Zhang, Can. J. Chem., 2009, 87, 1013 CrossRef CAS.
- M. Harb, F. Rabilloud, D. Simon, A. Rydlo, S. Lecoultre, F. Conus, V. Rodrigues and C. Félix, J. Chem. Phys., 2008, 129, 194108 CrossRef CAS PubMed.
- M. Yang, K. A. Jackson and J. Jellinek, J. Chem. Phys., 2006, 125, 144308 CrossRef CAS PubMed.
- K. Michaelian, N. Rendón and I. L. Garzón, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 60, 2000 CrossRef CAS.
- S. Debnath and S. M. Said, Structural and electronic properties of Li doped decahedral Ag12Li1 bimetallic nanoclusters, Semiconductor Electronics (ICSE), 2014 IEEE International Conference on. IEEE, 2014, p. 278.
- J. S. Pilgrim and M. A. Duncan, Chem. Phys. Lett., 1995, 232, 335 CrossRef CAS.
- A. Stangassinger, A. M. Knight and M. A. Duncan, Chem. Phys. Lett., 1997, 266, 189 CrossRef CAS.
- C. S. Yeh, D. L. Robbins, J. S. Pilgrim and M. A. Duncan, Chem. Phys. Lett., 1993, 206, 509 CrossRef CAS.
- G. Mie, Ann. Phys., 1908, 25, 377 CrossRef CAS.
- B. T. Draine and P. J. Flatau, J. Opt. Soc. Am. A, 1994, 11, 1491 CrossRef.
- K. S. Kunz and R. J. Luebbers, The finite difference time domain method for electromagnetics, CRC press, 1993 Search PubMed.
- M. A. Marques, N. T. Maitra, F. M. Nogueira, E. K. Gross and A. Rubio, Fundamentals of time-dependent density functional theory, Springer, 2012 Search PubMed.
- W. Ma and F. Chen, J. Alloys Compd., 2012, 541, 79 CrossRef CAS PubMed.
- W. Ma and F. Chen, J. Mol. Model., 2013, 19, 4555 CrossRef CAS PubMed.
- Y. Rao, Y. Lei, X. Cui, Z. Liu and F. Chen, J. Alloys Compd., 2013, 565, 50 CrossRef CAS PubMed.
- B. Delley, J. Chem. Phys., 1990, 92, 508 CrossRef CAS PubMed.
- B. Delley, J. Chem. Phys., 2000, 113, 7756 CrossRef CAS PubMed.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865 CrossRef CAS.
- B. Delley, Phys. Rev. B: Condens. Matter Mater. Phys., 2002, 66, 155125 CrossRef.
- E. K. U. Gross and W. Kohn, in Advances in Quantum Chemistry, ed. L. Per-Olov, Academic Press, 1990, p. 255 Search PubMed.
- J. Emsley, The Elements, Clarendon Press Oxford, 2nd edn, 1991 Search PubMed.
- C. Kittel, Introduction to Solid State Physics, 8th edn, 2005 Search PubMed.
- J.-i. Aihara, J. Phys. Chem. A, 1999, 103, 7487 CrossRef CAS.
- D. E. Manolopoulos, J. C. May and S. E. Down, Chem. Phys. Lett., 1991, 181, 105 CrossRef CAS.
- L. M. Molina and B. Hammer, J. Catal., 2005, 233, 399 CrossRef CAS PubMed.
- H.-C. Weissker and C. Mottet, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 84, 165443 CrossRef.
- X. Cai, P. Zhang, L. Ma, W. Zhang, X. Ning, L. Zhao and J. Zhuang, J. Phys. Chem. A, 2009, 113, 4889 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |