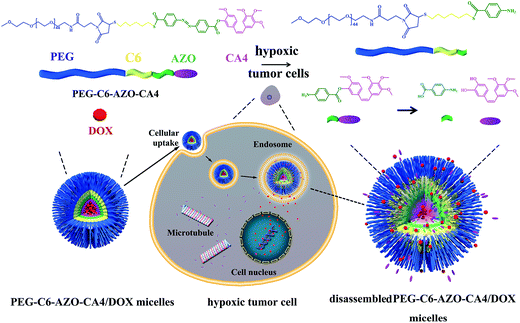
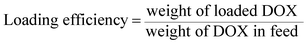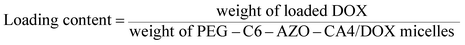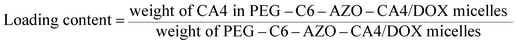Development of hypoxia-triggered prodrug micelles as doxorubicin carriers for tumor therapy†
Hongmei Liu‡
bc,
Ruilong Zhang‡bd,
Yunwei Niua,
Yan Libc,
Chenmeng Qiaoe,
Jie Wenge,
Jun Lif,
Xiaoning Zhang*f,
Zuobing Xiao*a and
Xin Zhang*ab
aSchool of Perfume and Aroma Technology, Shanghai Institute of Technology, Shanghai, 200233, China. E-mail: xzb@sit.edu.cn; xzhang@ipe.ac.cn
bNational Key Laboratory of Biochemical Engineering, Institute of Process Engineering, Chinese Academy of Sciences, Beijing, 100190, China
cUniversity of Chinese Academy of Sciences, Beijing, 100049, China
dInstitute of Materials Science and Engineering, Ocean University of China, Qingdao, Shandong Province 266100, China
eKey Laboratory of Advanced Technologies of Materials, School of Materials Science and Engineering, Southwest Jiaotong University, Chengdu, Sichuan 610031, China
fCollaborative Innovation Center for Biotherapy, School of Medicine, Tsinghua University, Beijing 100084, China. E-mail: drugman@tsinghua.edu.cn
First published on 23rd January 2015
Abstract
Hypoxia has a major role in tumor development and resistance to therapy. Therefore, the effective targeting and killing of hypoxic tumor cells is a key to successful tumor control. Here, we report the hypoxia-responsive prodrug micelles to deliver hydrophobic anticancer drug, which can selectively release the drugs to treat hypoxic tumor cells in a combined way. For this purpose, an azobenzene (AZO) bond, which imparts hypoxia sensitivity and specificity as cross linker, conjugated PEG–hexanethiol (PEG–C6) with combretastatin A-4 (CA4) to form PEG–C6–AZO–CA4 amphiphilic molecule. These PEG–C6–AZO–CA4 molecules self-assemble into micelles, which can encapsulate hydrophobic anticancer drug. The drug release behavior from PEG–C6–AZO–CA4 micelles was studied under normoxic or hypoxic conditions and the combinations of CA4 with hydrophobic drugs for tumor treatment in vitro were also investigated. As the first example of using AZO linkages to develop anticancer prodrug micelles as hydrophobic anticancer drugs delivery to kill the hypoxic tumor cells in a combination way, this study establishes PEG–C6–AZO–CA4 micelles as a promising drug delivery platform for hypoxic tumor therapy.
1. Introduction
Hypoxia is a salient feature of solid tumors and the levels of hypoxia are more severe in most tumors than normal tissues.1–4 In some tumors, the tumor tissues partial pressures of oxygen are near zero mm Hg, compared with the normal tissues (∼30 mm Hg).5 Clinical studies have demonstrated that patients with hypoxic tumors (≤10 mm Hg) have significantly low survival rate.6,7 This is because hypoxic tumors are often resistant to conventional cancer therapies.8 Therefore, effective targeting and killing of hypoxic cells is a key to successful tumor control. For example, bioreductive prodrugs, such as SN29428, TH302, and SN29966, had been developed for hypoxic tumor treatment.9–12 However, most of bioreductive prodrugs generate DNA-reactive cytotoxins, making them difficult to combine with conventional chemotherapy because of overlapping toxicity. On the other hand, bioreductive prodrugs are confronted with substantial challenge in drug penetration into hypoxic zones. The penetration problem is particularly severe and has largely been ignored for bioreductive prodrugs, many of which are designed to be metabolized as they diffuse into hypoxic zones.2Nanoparticles with different materials can deliver drugs with different mechanisms to tumor and be preferential accumulation in tumor tissues via the enhanced permeation and retention (EPR) effect.13,14 However, it is difficult to develop nanoparticle that can release drugs selectively under hypoxia. Therefore, a smart hypoxia-responsive linkage was employed for the release of drugs under hypoxia, which can contribute significantly to the successful treatment of the hypoxic tumor cells.
Most of the hypoxia-sensitive moiety contains nitroaryl or a quinone group.15–21 Recently, an AZO group was reported that has excellent properties as the hypoxia-sensitive moiety and was widely used in the development of imaging agents and bioreductive prodrugs for selectively hypoxic tumors.22,23 Kenjiro Hanaoka and co-workers developed AZO-based fluorescent probes that made use of the reduction of an AZO group in the reductive environment of hypoxia.24 Alan C. Sartorelli and co-workers synthesized hypoxia-selective O6-alkylguanine DNA alkyltransferase inhibitors by AZO linkage, which inhibited the survival of DU145 cells effectively by the combination of laromustine.25 Currently, most researchers conjugated an AZO moiety directly to a fluorophore to quench the fluorescence or to chemical drug to target hypoxic tumor environment. However, there are no studies available for targeted drug delivery systems using AZO cross linked hypoxia-responsive prodrug micelles.
Herein, we have successfully developed AZO-based micelles for hydrophobic anticancer drug delivery to kill hypoxic tumor cells in a combined way and overcome the abovementioned difficulties of killing hypoxic tumor cells (Scheme 1). CA4 was conjugated to a PEG–C6 through an AZO linker to form PEG–C6–AZO–CA4 amphiphilic molecules. These PEG–C6–AZO–CA4 amphiphilic molecules can self-assemble into AZO-based micelles in an aqueous solution, which was named PEG–C6–AZO–CA4 micelles. Due to the introduction of AZO linkage in PEG–C6–AZO–CA4 micelles, it was expected to disassemble the micelles under the reductive environment of hypoxia. The PEG–C6–AZO–CA4 amphiphilic molecules are composed of four components: (1) PEG part, (2) C6 part, (3) AZO bond and (4) CA4 part. According to our design, PEG as hydrophilic part was expected to prolong PEG–C6–AZO–CA4 micelles circulation time and escape from immunological recognition.26 Hydrophobic C6 was chosen as a crosslinker to increase the hydrophobic proportion of PEG–C6–AZO–CA4. Hydrophobic AZO bond was used because of its reductive cleavage.22–25 We hoped that when the AZO bond is cleaved, the proportion of hydrophobic and hydrophilic micelles is changed, resulting in the disassembly of micelles to release the embedded drugs faster. CA4 is one of the most important natural molecules that strongly inhibits tubulin polymerization to kill the tumor cells.27–29 We chose anticancer drug CA4 for a prodrug design because of its simple structure with a phenolic group, hydrophobic feature and its potent cytotoxicity.
Considering the components of PEG–C6–AZO–CA4 molecules, we hypothesized that self-assembled PEG–C6–AZO–CA4 micelles might exhibit the following advantages: (1) encapsulate hydrophobic drugs (e.g., doxorubicin (DOX)) to kill tumors in combined effects, (2) release CA4 and hydrophobic drug rapidly when they were accumulated to hypoxic zone of tumor, (3) form stable and compact structure to enhance the colloidal stability. To test this hypothesis, encapsulated hydrophobic anticancer drug (e.g., DOX) PEG–C6–AZO–CA4 micelles (PEG–C6–AZO–CA4/DOX micelles) were prepared. The drug release behaviors of PEG–C6–AZO–CA4/DOX micelles were evaluated under normoxic or hypoxic conditions. In addition, we examined the DOX biodistribution and synergistic therapeutic effects in MCF-7 cells. Therefore, it is important for the developed hypoxia-sensitive prodrug carrier to co-deliver conventional chemotherapy to cure hypoxic tumor in combination ways.
2. Experimental
2.1. Materials
4,4′-Azobenzenedicarboxylic acid, oxalyl chloride and 1,6-hexanedithiol were purchased from J&K Scientific. Triethylamine was purchased from Alfa Aesar. DOX·HCl and combretastatin A-4 (CA4) were obtained from Dalian Meilun Biotech Co. Ltd., (Dalian, China). Hoechst 33258, catalase and glucose oxidase from Aspergillus niger were obtained from TCI Shanghai. PEG-MAL2000 was obtained from Shanghai Yare Biotech. Dichloromethane was dried by calcium hydride. Doxorubicin hydrochloride (DOX·HCl) was deprotonated with triethylamine in dimethyl sulphoxide (DMSO) to obtain the hydrophobic DOX.2.2. Synthesis of C6–AZO–CA4
4,4′-Azobenzenedicarboxylic acid (100 mg, 0.37 mmol) was dissolved in anhydrous CH2Cl2 by adding a drop anhydrous DMF. Oxalyl chloride (125 μL) was dissolved in 10 mL of anhydrous CH2Cl2 and was added dropwisely into the 4,4′-azobenzenedicarboxylic acid solution. The reaction was carried out for 2 h at room temperature. The resultant solution was dried under vacuum. The obtained product was 4,4′-dichloroformylazobenzene. Then, CA4 (117 mg, 0.37 mmol) and pyridine (87 μL, 1.11 mmol) were dissolved in 10 mL of anhydrous CH2Cl2 and was added dropwisely into 4,4′-dichloroformylazobenzene solution for 1 h. 1,6-Hexanedithiol (59 μL, 0.37 mmol) and pyridine (87 μL, 1.11 mmol) were dissolved in 10 mL of anhydrous CH2Cl2 and was added dropwisely into a solution of CA4-4 and 4′-dichloroformylazobenzene for 2 h. The crude product was purified by column chromatography on silica gel (200–300 mesh) eluted with 0–10% methanol gradient in dichloromethane, which gave C6–AZO–CA4 as a red solid.2.3. Synthesis of PEG–C6–AZO–CA4
C6–AZO–CA4 (100 mg, 0.143 mmol) and mPEG-Mal (286 mg, 0.143 mmol) were dissolved in anhydrous CH2Cl2 with gentle stirring for 4 h at room temperature. 1H NMR (600 MHz, CDCl3 δ ppm) was carried out to characterize the PEG–C6–AZO–CA4.2.4. Preparation of PEG–C6–AZO–CA4 micelles and PEG–C6–AZO–CA4/DOX micelles aqueous solution
The PEG–C6–AZO–CA4 molecules (4 mg) were dissolved in DMSO (200 μL) and then added dropwisely into 1 mL of deionized water under strong stirring. This solution was then dialyzed against deionized water for 1 day to remove DMSO. The PEG–C6–AZO–CA4 molecules (4 mg) and DOX (300 μg) were dissolved in DMSO (200 μL) and then added dropwisely into 1 mL of deionized water under vigorous stirring. This solution was then dialyzed against deionized water for 1 day to remove DMSO.2.5. The morphologies of PEG–C6–AZO–CA4 micelles, PEG–C6–AZO–CA4/DOX micelles, disassembled PEG–C6–AZO–CA4 micelles and PEG–C6–AZO–CA4/DOX micelles
The morphologies of the micelles were observed by cryoelectron microscopy (FEI Tecnai 20 cryo-TEM).To examine whether these micelles can disassemble in hypoxia biological systems, we first conducted an in vitro assay using rat liver microsomes, which contain various reductases. After adding NADPH (50 μM) as a cofactor for the reductases to rat liver microsomes (75 mg mL−1), which containing the aqueous solution of PEG–C6–AZO–CA4 micelles or PEG–C6–AZO–CA4/DOX micelles, the morphology of the disassembled micelles was observed by cryo-TEM.24
2.6. Size, zeta potential and colloidal stability of micelle measurements
Micellar size and zeta potential were determined using a Malvern Zetasizer NanoZS. Colloidal stability was measured by incubating PEG–C6–AZO–CA4/DOX micelles in Dulbecco's modified Eagle's medium (DMEM) with high glucose supplemented with 10% fetal bovine serum (FBS) at 37 °C under gentle stirring. At each time point, the mean diameters of micelles were monitored by dynamic light scattering (DLS).2.7. CA4 equivalent loading, DOX loading efficiency and in vitro release of CA4 and DOX from PEG–C6–AZO–CA4/DOX micelles
The weight of loaded DOX in micelles was measured by a microplate reader (SpectraMax M5, Molecular Devices, CA) with excitation at 470 nm and emission at 590 nm after the micelles were dissolved in DMSO. The total weight of PEG–C6–AZO–CA4/DOX micelles was calculated by adding the weight of PEG–C6–AZO–CA4 molecules used for the preparation of the micelles and the weight of loaded DOX. The loading efficiency and loading content of DOX were calculated as follows:30The weight of CA4 in micelles was calculated by the relative molecular weight between CA4 and PEG–C6–AZO–CA4 molecules. The loading efficiency of CA4 was calculated using the following equation:31
The loading content of CA4 into the PEG–C6–AZO–CA4/DOX micelles was found to be 10.9 wt%. The loading efficiency and loading content of DOX into the PEG–C6–AZO–CA4/DOX micelles was found to be 95.2% and 6.7 wt%, respectively.
The release profiles of DOX from PEG–C6–AZO–CA4/DOX micelles were studied using a dialysis tube (MWCO 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) under shaking (200 rpm) at 37 °C in PBS (pH 7.4). The amount of DOX released was determined by a microplate reader (SpectraMax M5, Molecular Devices, CA) with excitation at 470 nm and emission at 590 nm.
000) under shaking (200 rpm) at 37 °C in PBS (pH 7.4). The amount of DOX released was determined by a microplate reader (SpectraMax M5, Molecular Devices, CA) with excitation at 470 nm and emission at 590 nm.
2.8. Enzymatic micelle disassembly process
To examine whether these micelles can disassemble in hypoxia biological systems, we prepared PEG–C6–AZO–CA4 micelles embedded hydrophobic DOX. The hydrophobic DOX is released and gradually subsided when the micelles were disassembled in simulation hypoxic environment. The simulation hypoxic environment was constructed using rat liver microsomes (75 mg mL−1) as a reductase and NADPH (50 μM) as a cofactor to consume the oxygen. After adding the rat liver microsomes and NADPH mixture to the PEG–C6–AZO–CA4/DOX micelles solution, the oxygen content was depleted rapidly to near zero in approximately 3 min, whereas the oxygen content was about 21% in the normoxic condition.32 After few minutes, these solutions were imaged by camera.The rat liver microsomes (75 mg mL−1) and NADPH (50 μM) were mixed and added to PEG–C6–AZO–CA4 micelles solution to cleave AZO bond. Then, the disassembled PEG–C6–AZO–CA4 micelles solution was freeze-dried and dissolved in DMSO. UV-vis absorption spectrum of PEG–C6–AZO–CA4 molecule and cleavage AZO bond of PEG–C6–AZO–CA4 molecule, which were dissolved in DMSO, was measured by UV-vis spectrophotometer (TU1810).
2.9. Enzymatic micelles disassembly process in MCF-7 cells
PEG–C6–AZO–CA4 micelles embedding DOX was prepared as described above. MCF-7 cells (4 × 104 cells per well) were seeded in a 35 mm glass bottom culture dish, incubated for 24 h at 37 °C in 5% CO2, and allowed to grow until 50% confluent. By adding PEG–C6–AZO–CA4 micelles to 35 mm glass bottom culture dish under normoxic or hypoxic, the cells on culture dish were observed by live cell station. The condition of hypoxia in cells was generated by the direct depletion of oxygen in sealed environment using the glucose oxidase (2 units per mL) and catalase (120 units per mL) dual enzyme system as previously described.32 All images were collected with UltraVIEW® VoX 3D live cell imaging system (PerkinElmer). For time-lapse experiments, images were collected every 30 s for about 60 min.To investigate the intracellular drug release from micelles, a density of 1 × 105 MCF-7 cells were plated into 35 mm Petri dish after 24 hours incubation, and then, the cells were incubated with micelles under normoxic or hypoxic conditions for 4 h. For nuclear staining, the cells were incubated with Hoechst 33285 for 10 min at 37 °C and then washed by PBS (pH 7.4) twice. The intracellular localization of DOX released from micelles was observed by confocal laser scanning microscopy (CLSM).
2.10. Drug toxicity studies
Clonogenic survival assays were performed as described previously.32 MCF-7 cells were plated into plastic 25 cm2 tissue culture flasks at a density of 5 × 105 cells and grown for 24 h. The cells were pretreated for 1 h without drug in the presence of glucose oxidase (2 units per mL), catalase (120 units per mL) and 10 mM of added glucose to generate hypoxic condition. After pretreatment, PEG–C6–AZO–CA4 micelles or PEG–C6–AZO–CA4/DOX micelles with two different concentrations were added, and the treatment continued for an additional 2 h. After treatment, the cells were detached by trypsinization, suspended in culture medium and counted. Cells were plated into six-well plates at a density of 1000 cells per well. Seven days later, colonies were fixed, stained with crystal violet, and counted.3. Results and discussion
3.1. Synthesis and characterization of PEG–C6–AZO–CA4
To test the aforementioned hypotheses, the PEG–C6–AZO–CA4 molecule was synthesized. The synthetic routes and chemical structures of PEG–C6–AZO–CA4 are given in Fig. 1A. The formation of C6–AZO–CA4 was first confirmed by 1H-NMR spectroscopy with all the characteristic peaks and the integration values of C6–AZO–CA4, as indicated in Fig. 1B (1H NMR 600 MHz, CDCl3 δ ppm) was carried out to characterize the C6–AZO–CA4 (46.5 mg, 17.09%). 1H NMR spectroscopy (600 MHz, CDCl3 δ ppm) was carried out to characterize the C6–AZO–CA4: 1.34 (–SH, t, J = 7.7 Hz, 1H), 1.46–1.47 (HS–CH2–CH2–(CH2)2–CH2–, m, 4H), 1.63–1.66 (HS–CH2–CH2–CH2–, m, 2H), 1.71–1.73 (CH2–CH2–CH2–S–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, m, 2H), 2.50–2.56 (HS–CH2–CH2, dt, J = 7.3 Hz, 7.5 Hz, 2H), 3.10–3.13 (CH2–CH2–S–C
O, m, 2H), 2.50–2.56 (HS–CH2–CH2, dt, J = 7.3 Hz, 7.5 Hz, 2H), 3.10–3.13 (CH2–CH2–S–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, t, J = 7.3 Hz, 2H), 3.73 (Ph–O–CH3, s, 6H), 3.81 (Ph–O–CH3, s, 3H), 3.82 (Ph–O–CH3, s, 3H), 6.46–6.54 (Ph–CH
O, t, J = 7.3 Hz, 2H), 3.73 (Ph–O–CH3, s, 6H), 3.81 (Ph–O–CH3, s, 3H), 3.82 (Ph–O–CH3, s, 3H), 6.46–6.54 (Ph–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–Ph, m, 2H), 6.54 (CH3O–Ph(a,e)–H, s, 2H), 6.91–6.92 (CH3O–Ph(i)–H, d, J = 8.5 Hz, 1H), 7.15 (O
CH–Ph, m, 2H), 6.54 (CH3O–Ph(a,e)–H, s, 2H), 6.91–6.92 (CH3O–Ph(i)–H, d, J = 8.5 Hz, 1H), 7.15 (O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O–CH3O–Ph(k)–H, d, J = 1.8 Hz, 1H), 7.18–7.19 (Ph(h)–H, dd, J = 1.8 Hz, 8.5 Hz, 1H), 8.01–8.03 (–Ph(n,o,p,q)–H, m, 4H), 8.13–8.14 (–Ph(r,s)–H, m, 2H), 8.32–8.33 (–Ph(l,m)–H, m, 2H). The mass and molecular formula of C6–AZO–CA4 were determined by Fourier transform ion cyclotron resonance mass spectrometer (positive, Bruker, USA) m/z 701.23 [M + H]+, 718.23 [M + NH4]+; calcd for C38H40N2O2S2: 700.23, found: 701.23 (Fig. S1†). As shown in Fig. S1,† the result was consistent with the expected formula of the C6–AZO–CA4. These data indicated that C6–AZO–CA4 was synthesized. The C6–AZO–CA4 was then coupled with PEG-Mal2000 to form PEG–C6–AZO–CA4 molecule. The formation of PEG–C6–AZO–CA4 was first confirmed by 1H-NMR spectroscopy. The reaction at the maleimide group of PEG was confirmed by the disappearance of double bond of PEG-Mal at δ 6.94 ppm and the appearance the B position of PEG–C6–AZO–CA4 at δ 3.47 ppm. The PEG peak of PEG–C6–AZO–CA4 molecule appeared at δ 3.64 ppm (Fig. 1C).
C–O–CH3O–Ph(k)–H, d, J = 1.8 Hz, 1H), 7.18–7.19 (Ph(h)–H, dd, J = 1.8 Hz, 8.5 Hz, 1H), 8.01–8.03 (–Ph(n,o,p,q)–H, m, 4H), 8.13–8.14 (–Ph(r,s)–H, m, 2H), 8.32–8.33 (–Ph(l,m)–H, m, 2H). The mass and molecular formula of C6–AZO–CA4 were determined by Fourier transform ion cyclotron resonance mass spectrometer (positive, Bruker, USA) m/z 701.23 [M + H]+, 718.23 [M + NH4]+; calcd for C38H40N2O2S2: 700.23, found: 701.23 (Fig. S1†). As shown in Fig. S1,† the result was consistent with the expected formula of the C6–AZO–CA4. These data indicated that C6–AZO–CA4 was synthesized. The C6–AZO–CA4 was then coupled with PEG-Mal2000 to form PEG–C6–AZO–CA4 molecule. The formation of PEG–C6–AZO–CA4 was first confirmed by 1H-NMR spectroscopy. The reaction at the maleimide group of PEG was confirmed by the disappearance of double bond of PEG-Mal at δ 6.94 ppm and the appearance the B position of PEG–C6–AZO–CA4 at δ 3.47 ppm. The PEG peak of PEG–C6–AZO–CA4 molecule appeared at δ 3.64 ppm (Fig. 1C).
 | ||
| Fig. 1 Synthesis of PEG–C6–AZO–CA4. (A) Synthetic route of the PEG–C6–AZO–CA4. (B) 1H NMR spectra of C6–AZO–CA4 (600 MHz, CDCl3). (C) 1H NMR spectra of PEG–C6–AZO–CA4 (600 MHz, CDCl3). | ||
3.2. Preparation and characterization of PEG–C6–AZO–CA4 micelles
After the successful synthesis of the PEG–C6–AZO–CA4 molecules, self-assembly and encapsulating hydrophobic drugs abilities of PEG–C6–AZO–CA4 were evaluated. In this study, DOX was chosen as model anticancer drug with two features. First, DOX causes DNA damage and the induction of apoptosis by the inhibition of the progression of the enzyme topoisomerase II.33 CA4 is one of the most important natural molecules that strongly inhibit tubulin polymerization. Previous research has shown that combined DOX with CA4 to cure tumor got effective results.34,35 In this study, we used PEG–C6–AZO–CA4 micelles to encapsulate DOX to cure hypoxic tumor in a combined way. Second, DOX had auto-fluorescence, which was easy to reveal micelles' properties and the interaction between the drug and micelle. The PEG–C6–AZO–CA4 micelles and PEG–C6–AZO–CA4/DOX micelles were prepared and characterized by cryo-TEM and DLS. As shown in Fig. 2A and B, PEG–C6–AZO–CA4 micelles and PEG–C6–AZO–CA4/DOX micelles were spherical micelles with unimodal size distribution by cryo-TEM. An average diameter of PEG–C6–AZO–CA4 micelles was about 65.9 ± 4.3 nm in an aqueous solution, which was determined by DLS studies. PEG–C6–AZO–CA4 micelles encapsulated DOX with a particle size of 75.9 ± 4.9 nm were bigger than PEG–C6–AZO–CA4 micelles because they were embedded with DOX (Fig. 2C). The particle size of micelles (20–100 nm) was beneficial for tumor targeting delivery, which was large enough to avoid renal filtration and lymphatic clearance and small enough to penetrate through the leaky vasculatures in tumor region.36 Therefore, PEG–C6–AZO–CA4/DOX micelles were easy to accumulate in tumor region. The equivalent CA4 loading efficiency of PEG–C6–AZO–CA4/DOX micelles was 10.9 wt%. The loading efficiency and loading content of DOX into the PEG–C6–AZO–CA4/DOX micelles was found to be 95.2% and 6.7 wt%, respectively. The PEG–C6–AZO–CA4/DOX micelles reduced the amount of inert materials to embed DOX and enhanced the CA4 loading efficiency due to conjugated CA4 as a component of micelle.Due to the introduction of hypoxia-sensitive AZO bonds, the PEG–C6–AZO–CA4 micelles and PEG–C6–AZO–CA4/DOX micelles were expected to disassemble quickly when they are exposed to reductive enzymes under the conditions of hypoxia. To examine whether PEG–C6–AZO–CA4 micelles and PEG–C6–AZO–CA4/DOX micelles can disassemble by reductive enzymes under conditions of hypoxia, we first prepared PEG–C6–AZO–CA4 micelles and PEG–C6–AZO–CA4/DOX micelles and conducted an in vitro assay in rat liver microsomes, which contain various reductases, mixed with NADPH.24 After adding NADPH (50 μM) as a cofactor for the reductases to yellow PEG–C6–AZO–CA4 prodrug micelles aqueous solution (4 mg mL−1) in the presence of rat liver microsomes (75 μg mL−1) (Fig. 2Da1), the yellow solution slowly turned colorless and a yellow solid appeared in centrifuge tube (Fig. 2Da2). Moreover, the red PEG–C6–AZO–CA4/DOX micelles aqueous solution (4 mg mL−1) (Fig. 2Db1) slowly turned transparent and the red precipitate was present (Fig. 2Db2) under the same hypoxic condition with PEG–C6–AZO–CA4 micelles. These results suggested that the micelles solution under reductive hypoxic condition resulted in the cleavage of the AZO bonds and released the drugs. To further confirm the disassembly of micelles under reductive hypoxic condition, the morphologies of PEG–C6–AZO–CA4 micelles and PEG–C6–AZO–CA4/DOX micelles after treatment with NADPH and rat liver microsomes were observed by cryo-TEM. From Fig. 2E and F, the size of PEG–C6–AZO–CA4 micelles and PEG–C6–AZO–CA4/DOX micelles gradually became bigger, some micelles disassembled and the number of micelles decreased. DLS analysis further confirmed these conclusions that the size and the polydispersity index (PDI) of PEG–C6–AZO–CA4 micelles and PEG–C6–AZO–CA4/DOX micelles became bigger after the enzyme treatment for 2 min (Fig. 2G and H). The counting rate of PEG–C6–AZO–CA4 micelles and PEG–C6–AZO–CA4/DOX micelles were decreased from 350 to 119 and from 399 to 149, respectively. These results coincided with the cryo-TEM observed and showed that the number of micelles was decreased. The cleavage of AZO bond mechanism of PEG–C6–AZO–CA4 molecule is shown in Fig. 2I. AZO bond conjugated with different groups will lead to a significant red shift of the π–π* band.37 As shown in Fig. 2J, PEG–C6–AZO–CA4 molecule had a broad absorption band centered at 450 nm, which is typical for the AZO chromophore. This absorption range disappeared when the AZO bond of PEG–C6–AZO–CA4 molecule was cleaved. Therefore, PEG–C6–AZO–CA4 micelles and PEG–C6–AZO–CA4/DOX micelles were disassembled and triggered the fast release of encapsulated DOX and free CA4 under the conditions of reductive enzymes in hypoxia.
The in vitro colloidal stability of PEG–C6–AZO–CA4 micelles and PEG–C6–AZO–CA4/DOX micelles was investigated in DMEM containing 10% FBS. As shown in Fig. 3A, for 72 hours incubation of PEG–C6–AZO–CA4 micelles and PEG–C6–AZO–CA4/DOX micelles, no significant size changes were observed. These results suggested that PEG–C6–AZO–CA4 micelles and PEG–C6–AZO–CA4/DOX micelles had better colloidal stability in FBS. The release of DOX from PEG–C6–AZO–CA4/DOX micelles was studied in PBS (pH 7.4) at 37 °C. The result showed that 12.2% of DOX was released in 4 days at pH 7.4 PBS (Fig. 3B). This result indicated that PEG–C6–AZO–CA4/DOX micelles are sufficiently stable under physiological conditions, whereas the red solution turned colorless and a red solid appeared in the presence of double enzyme, as shown in the Fig. 2Db. Therefore, these findings are important because colloidal stability in FBS and slow release of the drug from micelles in physiological environment for any nanoparticles (NPs) determines the successful delivery of drugs to target position as it prevents particle aggregation or embolism and premature burst drug release from circulatory system and prolongs the NPs residence time in the body.38
 | ||
| Fig. 3 (A) The stability of PEG–C6–AZO–CA4 micelles and PEG–C6–AZO–CA4/DOX micelles in DMEM with 10% FBS (n = 3). (B) Release profiles of DOX from PEG–C6–AZO–CA4/DOX micelles (n = 3). | ||
3.3. Characterization of PEG–C6–AZO–CA4 micelles in hypoxic tumor cells
In order to examine the release profiles of micelles under normoxic or hypoxic tumor cells, we applied PEG–C6–AZO–CA4 micelles and PEG–C6–AZO–CA4/DOX micelles to living tumor cells (MCF-7). Nano-environment induces self-organization of DOX molecules driven by their intermolecular π–π stacking interaction.26,39–41 Therefore, when the DOX encapsulated into the micelles, the fluorescent signal of DOX molecules is quenched almost completely. Once released from the micelles, the fluorescence of DOX molecules is restored. We used this photophysical property of DOX to investigate the hypoxia-responsive activated drug release performance of PEG–C6–AZO–CA4/DOX micelles. The release behavior of DOX from PEG–C6–AZO–CA4/DOX micelles was observed using UltraVIEW® VoX 3D live cell imaging system. Almost no DOX fluorescence was observed in MCF-7 cells following 60 min incubation with PEG–C6–AZO–CA4/DOX micelles (2 mg mL−1) under normoxic conditions in merged images (Fig. 4Aa and Video S1 in the ESI†), whereas less DOX fluorescence was observed following 20 min, and at 60 min, the strongest DOX fluorescence appeared in merged image under hypoxic conditions (Fig. 4Ab and Video S2 in the ESI†). These results indicate that PEG–C6–AZO–CA4/DOX micelles release DOX rapidly and indicate that AZO bonds are cleaved under hypoxic conditions just for a few minutes. After the incubation of PEG–C6–AZO–CA4/DOX micelles for 4 h under normoxic condition in MCF-7 cells, less fluorescence appeared in cytoplasm and there was no DOX fluorescence in nuclei (Fig. 4Ba). Moreover, free DOX was trafficked to the cell nuclei and strong DOX fluorescence appeared in perinuclear under hypoxic condition (Fig. 4Bb). These findings are in good agreement with UltraVIEW® VoX 3D live cell imaging system results. Under normoxic condition, DOX release from PEG–C6–AZO–CA4/DOX micelles through hydrolysis of the ester linkage between CA4 and PEG–C6–AZO.42 From the UltraVIEW® VoX 3D live cell imaging system and CLSM results, we found that PEG–C6–AZO–CA4/DOX micelles tend to passively release DOX and are limited to release an effective drug concentration at desired time and anti-tumor efficacy under normoxic condition. This was likely due to the retarded hydrolysis and drug diffusion within the hydrophobic core.43 Moreover, PEG–C6–AZO–CA4/DOX micelles released DOX quickly and reached maximum tumor accumulation at the time point under hypoxic condition, which significantly improved therapeutic effects in hypoxic tumor. Overall, PEG–C6–AZO–CA4/DOX micelles were highly susceptible to hypoxic conditions and released DOX rapidly.To evaluate the CA4 release behavior of PEG–C6–AZO–CA4 micelles under normoxic or hypoxic conditions in MCF-7 cells, we performed time-lapse imaging of MCF-7 cells in micelles for 60 min. CA4, isolated from Combretum caffrum, binds to the colchicines binding site of β-tubulin.44,45 CA4 is also one of the most important natural molecules that strongly inhibits tubulin polymerization to kill the tumor cells.10 Some previous studies described that cells treated with CA4 would retract the cell margins, formed the thin retraction fibers and caused loss of cell–cell interaction.46,47 PEG–C6–AZO–CA4 micelles caused cells contraction, formed thin retraction fibers (red arrow) and caused loss of cell–cell interaction (white arrow) starting within 60 min of micelles addition under normoxic conditions (Fig. 4Ca), it also occurred in Fig. 4A, especially in the Video S1,† where the phenomenon is obvious. These results suggested that PEG–C6–AZO–CA4 micelles can release free CA4 to inhibit tubulin polymerization and release free CA4 quickly under hypoxic condition, due to the cleavability of AZO bond under hypoxic condition. These results coincided with the abovementioned DOX released behaviors.
3.4. In vitro cytotoxicity
To evaluate the anticancer efficiency of PEG–C6–AZO–CA4 micelles and PEG–C6–AZO–CA4/DOX micelles, the clonogenic assays against MCF-7 cells were tested under normoxic or hypoxic conditions. The PEG–C6–AZO–CA4 micelles alone also exhibited antitumor activity due to the presence of the anticancer CA4 moiety. As expected, the PEG–C6–AZO–CA4 micelles with CA4 and the PEG–C6–AZO–CA4 micelles embedded DOX were significantly more cytotoxic under hypoxic condition than they are under normoxic condition. Compared with the cytotoxicity of PEG–C6–AZO–CA4 micelles with CA4, the cytotoxicity of PEG–C6–AZO–CA4/DOX micelles was considerably lower, no matter what the condition was, due to a synergistic action of DOX and free CA4. Notably, the survival of MCF-7 cells was lowest after treatment with PEG–C6–AZO–CA4 micelles embedded DOX (30 μM equivalent CA4 concentration and 4.5 μM DOX concentration), which reduced viability by 7.6± 0.68% compared to control under hypoxic condition (Fig. 5). This observation was consistent with our findings on releasing DOX and free CA4 quickly under hypoxic tumor cells, which enhanced the anticancer efficacy.4. Conclusions
In summary, we developed AZO-based prodrug micelles (PEG–C6–AZO–CA4 micelles) for DOX delivery. AZO-based prodrug micelles have a built-in hypoxic trigger release mechanism to further enhance drug deposition in tumors and enhanced the anticancer activity. We have successfully demonstrated the PEG–C6–AZO–CA4 micelles released DOX and free CA4 quickly under hypoxic conditions. Moreover, the strong cytotoxicity of CA4 and DOX was examined in MCF-7 cells. This finding suggested that the PEG–C6–AZO–CA4 micelles had the potential for the delivery of two drugs to provide a promising nanomedicinal approach for cancer treatment.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grants no. 21304099, 51203162, 51103159, 51373177), the National High Technology Research and Development Program (Grants no. 2014AA020708, 2012AA022703, 2012AA020804), the Instrument Developing Project of the Chinese Academy of Sciences (Grant no. YZ201253, YZ201313), Open Funding Project of the National Key Laboratory of Biochemical Engineering (Grant no. Y22504A169). “Strategic Priority Research Program” of the Chinese Academy of Sciences, Grant no. XDA09030301-3 was also acknowledged.Notes and references
- M. W. Moyer, Nat. Med., 2012, 18, 636–637 CrossRef PubMed.
- W. R. Wilson and M. P. Hay, Nat. Rev. Cancer, 2011, 11, 393–410 CrossRef CAS PubMed.
- A. L. Harris, Nat. Rev. Cancer, 2002, 2, 38–47 CrossRef CAS PubMed.
- J. M. Brown and W. R. Wilson, Nat. Rev. Cancer, 2004, 4, 437–447 CrossRef CAS PubMed.
- M. Takasawa, R. R. Moustafa and J.-C. Baron, Stroke, 2008, 39, 1629–1637 CrossRef CAS PubMed.
- S. M. Evans and C. J. Koch, Cancer Lett., 2003, 195, 1–16 CrossRef CAS.
- P. Vaupel and A. Mayer, Cancer Metastasis Rev., 2007, 26, 225–239 CrossRef CAS PubMed.
- Y. Kim, Q. Lin, P. M. Glazer and Z. Yun, Curr. Mol. Med., 2009, 9, 425–434 CrossRef CAS.
- F. W. Hunter, J. K. Jaiswal, D. G. Hurley, H. S. Liyanage, S. P. McManawaya, Y. C. Gu, S. Richter, J. Wanga, M. Tercel, C. G. Print, W. R. Wilsona and F. B. Pruijn, Biochem. Pharmacol., 2014, 89, 224–235 CrossRef CAS PubMed.
- J. D. Sun, Q. Liu and J. L. Wang, Clin. Cancer Res., 2012, 18, 758–770 CrossRef CAS PubMed.
- J. S. Hu, D. R. Handisides, E. V. Valckenborgh, H. D. Raeve, E. Menu, I. V. Broek, Q. Liu, J. D. Sun, B. V. Camp, C. P. Hart and K. Vanderkerken, Blood, 2011, 116, 1524–1527 CrossRef PubMed.
- A. V. Patterson, J. Jaswail, S. P. Syddall, M. Abbattista, W. V. Leeuwen, M. Puryer, A. Thompson, A. Hsu, S. Mehta, A. Pruijn, G. L. Lu, F. Doñate, W. A. Denny, W. R. Wilson and J. B. Smaill, Mol. Cancer Ther., 2009, 8(suppl. 1), B76 Search PubMed.
- H. Liu, Y. Li, A. Mozhi, L. Zhang, Y. Liu, X. Xu, J. Xing, X. Liang, G. Ma, J. Yang and X. Zhang, Biomaterials, 2014, 35, 6519–6533 CrossRef CAS PubMed.
- H. Liu, C. Qiao, J. Yang, J. Weng and X. Zhang, J. Mater. Chem. B, 2014, 2, 5910–5924 RSC.
- Y. Liu, Y. Xu, X. Qian, J. Liu, L. Shen, J. Li and Y. Zhang, Bioorg. Med. Chem., 2006, 14, 2935–2941 CrossRef CAS PubMed.
- L. Cui, Y. Zhong, W. Zhu, Y. Xu, Q. Du, X. Wang, X. Qian and Y. Xiao, Org. Lett., 2011, 13, 928–931 CrossRef CAS PubMed.
- K. Xu, F. Wang, X. Pan, R. Liu, J. Ma, F. Kong and B. Tang, Chem. Commun., 2013, 49, 2554–2556 RSC.
- H. Komatsu, H. Harada, K. Tanabe, M. Hiraoka and S. Nishimoto, MedChemComm, 2010, 1, 50–53 RSC.
- S. Zhang, M. Hosaka, T. Yoshihara, K. Negishi, Y. Iida, S. Tobita and T. Takeuchi, Cancer Res., 2010, 70, 4490–4498 CrossRef CAS PubMed.
- E. Nakata, Y. Yukimachi, H. Kariyazono, S. Im, C. Abe, Y. Uto, H. Maezawa, T. Hashimoto, Y. Okamoto and H. Hori, Bioorg. Med. Chem., 2009, 17, 6952–6958 CrossRef CAS PubMed.
- Z. Li, X. Li, X. Gao, Y. Zhang, W. Shi and H. Ma, Anal. Chem., 2013, 85, 3926–3932 CrossRef CAS PubMed.
- J. Rao and A. Khan, J. Am. Chem. Soc., 2013, 135, 14056–14059 CrossRef CAS PubMed.
- F. Perche, S. Biswas, T. Wang, L. Zhu and V. P. Torchilin, Angew. Chem., Int. Ed., 2014, 53, 3362–3366 CrossRef CAS PubMed.
- W. Piao, S. Tsuda, Y. Tanaka, S. Maeda, F. Y. Liu, S. Takahashi, Y. Kushida, T. Komatsu, T. Ueno, T. Terai, T. Nakazawa, M. Uchiyama, K. Morokuma, T. Nagano and K. Hanaoka, Angew. Chem., Int. Ed., 2013, 52, 13028–13032 CrossRef CAS PubMed.
- R. Zhu, R. P. Baumann, P. G. Penketh, K. Shyam and A. C. Sartorelli, J. Med. Chem., 2013, 56, 1355–1359 CrossRef CAS PubMed.
- K. Y. Choi, H. Y. Yoon, J.-H. Kim, S. M. Bae, R.-W. Park, Y. M. Kang, I.-S. Kim, I. C. Kwon, K. Choi, S. Y. Jeong, K. Kim and J. H. Park, ACS Nano, 2011, 5, 8591–8599 CrossRef CAS PubMed.
- G. G. Dark, S. A. Hill, V. E. Prise, G. M. Tozer, G. R. Pettit and D. J. Chaplin, Combretastatin A-4, Cancer Res., 1997, 57, 1829–1834 CAS.
- M. Bio, P. Rajaputra, G. Nkepang, S. G. Awuah, A. M. L. Hossion and Y. You, J. Med. Chem., 2013, 56, 3936–3942 CrossRef CAS PubMed.
- M. Bio, P. Rajaputra, G. Nkepang and Y. You, J. Med. Chem., 2013, 57, 3401–3409 CrossRef PubMed.
- T. Thambi, V. G. Deepagan, H. Y. Yoon, H. S. Han, S.-H. Kim, S. Son, D.-G. Jo, C.-H. Ahn, Y. D. Suh, K. Kim, I. C. Kwon, D. S. Lee and J. H. Park, Biomaterials, 2014, 35, 1735–1743 CrossRef CAS PubMed.
- D. Nicolas, D. Fabienne, P. Vincent, S. Jean-Marc, B. Olivier, S. L. Cécile, H. Stephanie, S. S. Ulrich, G. Jean-François, M. B. Jacqueline and P. Véronique, Bioconjugate Chem., 2014, 25, 72–81 CrossRef PubMed.
- R. P. Baumann, P. G. Penketh, H. A. Seow, K. Shyam and A. C. Sartorelolli, Radiat. Res., 2008, 70, 651–660 CrossRef PubMed.
- S. Aroui, S. Brahim, M. D. Waard and A. Kenani, Cytotoxicity, Biochem. Biophys. Res. Commun., 2010, 391, 419–425 CrossRef CAS PubMed.
- Y. G. Wang, T. Y. Yang, X. Wang, W. B. Dai, J. C. Wang, X. Zhang, Z. Q. Li and Q. Zhang, J. Controlled Release, 2011, 149, 299–306 CrossRef CAS PubMed.
- S. Sengupta, D. Eavarone, I. Capila, G. Zhao, N. Watson, T. Kiziltepe and R. Sasisekharan, Nature, 2005, 436, 568–572 CrossRef CAS PubMed.
- M. Elsabahy and K. L. Wooley, Chem. Soc. Rev., 2012, 41, 2545–2561 RSC.
- U. A. Hrozhyk, S. V. Serak, N. V. Tabiryan, L. Hoke, D. M. Steeves, B. Kimball and G. Kedziora, Mol. Cryst. Liq. Cryst., 2008, 489, 257–272 CAS.
- A. Vanessa, P. H. Adriana, L. E. Magda, T. L. Madeline and R. Carlos, J. Nanopart. Res., 2013, 15, 1874–1888 CrossRef PubMed.
- E. Hayakawa, K. Furuya, H. Ueno, T. Kuroda, M. Moriyama and A. Kondo, Chem. Pharm. Bull., 1991, 39, 1009–1012 CrossRef CAS.
- M. K. Yu, Y. Y. Jeong, J. Park, S. Park, J. W. Kim, J. J. Min, K. Kim and S. Jon, Angew. Chem., Int. Ed., 2008, 120, 5442–5445 CrossRef.
- J.-H. Lee, K.-J. Chen, S.-H. Noh, M. A. Garcia, H. Wang, W. Y. Lin, H. Jeong, B. J. Kong, D. B. Stout, J. Cheon and H.-R. Tseng, Angew. Chem., Int. Ed., 2013, 52, 4384–4388 CrossRef CAS PubMed.
- R. Namgung, Y. M. Lee, J. Kim, Y. Jang, B.-H. Lee, I.-S. Kim, P. Sokkar, Y. M. Rhee, A. S. Hoffman and W. J. Kim, Nat. Commun., 2014, 5, 1–12 Search PubMed.
- F. X. Zhan, W. Chen, Z. J. Wang, W. T. Lu, R. Cheng, C. Deng, F. Meng, H. Liu and Z. Zhong, Biomacromolecules, 2011, 12, 3612–3620 CrossRef CAS PubMed.
- Y. Kong, J. Grembecka, M. C. Edler, E. Hamel, S. L. Mooberry, M. Sabat, J. Rieger and M. L. Brown, Chem. Biol., 2005, 12, 1007–1014 CrossRef CAS PubMed.
- Y. Jin, P. Qi, Z. Wang, Q. Shen, J. Wang, W. Zhang and H. Song, Molecules, 2011, 16, 6684–6700 CrossRef CAS PubMed.
- S. Kim, L. Peshkin and T. J. Mitchison, PLoS One, 2012, 7, e40177 CAS.
- T. J. Mitchison, Cell Motil. Cytoskeleton, 1992, 22, 135–151 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra14875d |
| ‡ H.L. and R.Z. contributed equally on this manuscript. |
| This journal is © The Royal Society of Chemistry 2015 |