Removal of cadmium and lead from aqueous solutions using nitrilotriacetic acid anhydride modified ligno-cellulosic material
Abstract
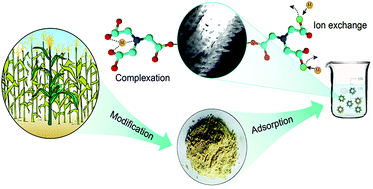
Cadmium (Cd2+) and lead (Pb2+) posed severe health risks worldwide. To remove these contaminants from aqueous solution, a nitrilotriacetic acid anhydride (NTAA) modified ligno-cellulosic material (NTAA-LCM) was prepared and characterized. Batch sorption experiments were performed to evaluate the influences of various factors such as contact time, pH, ionic strength, temperature and initial metal concentration on the sorption of metals. Results from elemental analysis and FTIR suggested that ester bonds and amine groups were successfully introduced into NTAA-LCM. Fast adsorption rates were observed, and the maximum sorption capacities of NTAA-LCM for Cd2+ and Pb2+ reached 143.4 and 303.5 mg g−1 at 298 K, respectively. Both the pseudo-second-order model and the Langmuir model described the adsorption extremely well. Thermodynamic analysis showed that the sorption was endothermic but spontaneous. The sorption was a chemical process involving surface chelation and ion exchange; this was reflected in the metal/NTA ratio. Additionally, NTAA-LCM retained high metal sorption capacity after seven cycles of regeneration by HNO3.

 Please wait while we load your content...
Please wait while we load your content...