Imidazolium salt-supported Mukaiyama reagent: an efficient condensation reagent for amide bond formation†
Khima Pandey,
Manoj Kumar Muthyala,
Sunita Choudhary and
Anil Kumar*
Department of Chemistry, Birla Institute of Technology and Science, Pilani, Rajasthan, India PIN-333031. E-mail: anilkumar@pilani.bits-pilani.ac.in; Fax: +91-1596-244183; Tel: +91-1596-245652
First published on 13th January 2015
Abstract
A novel imidazolium salt-supported Mukaiyama reagent (2-chloropyridinium salt) has been developed and explored as an efficient coupling agent for amide bond formation. The use of an ionic liquid-supported reagent enabled isolation of the amide products by simple extraction with organic solvents in high purity and avoiding column chromatography purification.
Introduction
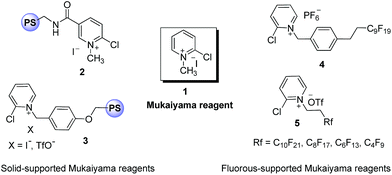
The amide bond is a significant building unit in many natural and synthetic compounds.1 The amide bond has a unique role in peptides and proteins. Molecules possessing amide bonds have shown a wide range of biological properties.2 Establishing synthetic methods for amides is an immensely important pursuit in organic synthesis. The most prevalent strategy for amide bond formation relies heavily upon the interconversion of activated carboxylic acid derivatives with an amine. However, other methodologies to access amide functionality have been also developed like the Staudinger reaction,3 hydrative amide syntheses with alkynes,4 the Schmidt reaction,5 the Beckmann rearrangement6 and amidation of aldehydes catalyzed by transition metals and hypervalent iodine reagents.7 These methods are highly efficient but have an innate drawback of using expensive transition metals, hazardous oxidants, longer reaction time and waste generation. Formation of amide bonds from carboxylic acids is not a spontaneous process at ambient temperature and generally it relies on activation of a carboxylic acid using a coupling reagent. To address these challenging problems several coupling and activating reagents such as 2-chloropyridinium salts, dicyclohexylcarbodiimide (DCC),8 1-ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline (EEDQ),9 the boron reagent B(OCH2CF3)3,10 nano-MgO11 and 1-[bis(dimethylamino)-methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium-3-oxide hexafluorophosphate (HATU) have been described in the literature for this reaction.12 Among these coupling reagents, N-methyl-2-chloropyridinium iodide (Mukaiyama reagent) (1) is an efficient activating agent for the formation of esters,13 carboxamides,14 ketenes,15 lactones16 and lactams17 from carboxylic acids. Despite its great success, it is also associated with some issues of solubility, stability and purification of coupled products.18 In most of the reactions using the Mukaiyama reagent, cumbersome chromatographic separation is mandatory to separate the product from the by-product, N-alkylpyridone. Over the last couple of years different approaches have been reported to address these problems associated with Mukaiyama reagents (Fig. 1). For example, Xu et al.19 have synthesized a novel N-ethyl-2-bromopyridinium tetrafluoroborate as an excellent coupling reagent for peptide synthesis with high activity and a low level of racemisation. The Tye,20 Taddei21 and Swinnen22 groups have independently developed polymer-supported Mukaiyama reagents (2 & 3) and demonstrated their application for the synthesis of carbodiimide, guanylation of primary amines, synthesis of amide & esters and generation of ketenes for Staudinger cycloaddition reactions, respectively. Although polymer-supported reagents have addressed some of the issues of 2-chloropyridinium salts, low loading capacity, high cost and slower reaction rates makes these approaches less attractive. Nagashima23 and Matsugi24 groups have independently reported fluorous tagged Mukaiyama reagents 4 and 5, respectively for the amide bond formation. The reactivity of reagent 5 was found to be dependent on the length of the fluorous tag in molecule.24b In the case of fluorous-supported synthesis, special solvent requirements and cost are serious concerns.25 Onium salt-supported reagents have gained considerable interest as a promising alternative soluble support for reagents owing to their high loading capacity, tunable solubility, homogeneity and easy monitoring of the reaction by various analytical techniques such as NMR, IR and mass spectrometry.26In continuation to our interest towards ionic liquid-supported reagents in organic synthesis,27 herein we report the synthesis of novel imidazolium salt-supported Mukaiyama reagent (2-chloropyridinium salt) and its application in amide bond formation. To the best of our knowledge this is the first report of the synthesis of an imidazolium salt-supported Mukaiyama reagents.
Result and discussion
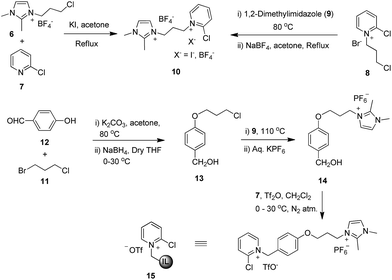
Our initial investigation to synthesize an imidazolium salt-supported Mukaiyama reagent by the reaction of 1-(3-chloropropyl)-2,3-dimethylimidazolium tetrafluoroborate (6) with 2-chloropyridine (7) in the presence of KI or by the reaction of 2-chloro-1-(3-chloropropyl)pyridine-1-ium bromide (8) with 1,2-dimethylimidazole (9) followed by anion exchange (Scheme 1) failed to give a substantial amount of imidazolium salt-supported Mukaiyama reagent 10.We envisioned the reaction of imidazolium salt-supported benzyl alcohol 14 with 2-chloropyridine (7) and triflic anhydride would afford us the desired imidazolium salt-supported Mukaiyama reagent.22 Synthesis of 14 was achieved from 4-hydroxy-benzaldehyde (12) as shown in Scheme 1. Reaction of 12 with 1-bromo-3-chloropropane (11) in the presence of potassium carbonate gave monoalkylated aldehyde, which on subsequent reduction resulted into the monoalkylated benzyl alcohol 13. Reaction of 13 with 1,2-dimethylimidazole (9) at 110 °C gave the corresponding chloride salt. Anion exchange of the chloride salt with aqueous KPF6 resulted in corresponding imidazolium salt-supported benzyl alcohol (14). Reaction of 14 and 7 in the presence of triflic anhydride under nitrogen atmosphere in dichloromethane for 10 h resulted in the imidazolium salt-supported Mukaiyama reagent 15 in good yield (55%). It is believed that sequential in situ formation of imidazolium salt-supported triflic acid ester followed by quaternization of 2-chloropyridine gave 15 (IL-supported Mukaiyama reagent). This reaction proved to be convenient and fast compared to our earlier attempts. The structure of reagent 15 was confirmed by 1H NMR, 13C NMR and HRMS (ESI†). Presence of two singlets at 3.73 ppm (NCH3) and 5.92 ppm (PhCH2), two doublets at 7.60 ppm and 7.64 ppm for the imidazolium protons along with characteristic double doublet at δ 9.27 for the ortho-protons adjacent to nitrogen of pyridinium and other peaks in the 1H NMR spectrum of 15 clearly indicated that the 2-chloropyridine has been tagged with imidazolium ion. Similarly, the number of peaks in the 13C NMR spectrum of 15 were well in agreement with the proposed structure. Presence of the peaks at m/z 502.4 [M-CF3SO3]+ and 506.3 [M − PF6]+ in the mass spectrum of 15 confirmed the formation of 15.
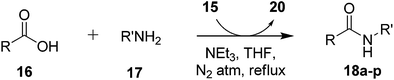
In order to explore synthetic applicability of the reagent, coupling of 4-nitrobenzoic acid (16) and 4-bromoaniline (17) was performed without additive in DCM as a prototype reaction. DCM was the first solvent of choice and this proved to work efficiently showing completion of reaction within 2 h. However, the choice of DCM was not optimal, because many carboxylic acids are insoluble in it, while it is too volatile for parallel synthetic use. Various solvents were screened to circumvent the issue. Among the various solvents evaluated for the reaction, THF proved to be the best choice in terms of yield and reaction time (Table 1, entry 4). Although the reagent was soluble in acetonitrile the yield of product was less even after longer reaction times. In methanol, the yield of the desired product was poor, probably because the nucleophilicity of the methanol also lead to the formation of the ester as a by-product.
Having identified optimum reaction conditions, the scope of reagent was examined by employing different substituted carboxylic acids and amines and results are summarized in Table 2. For the synthesis of amide, a mixture of benzoic acid (16), imidazolium salt-supported Mukaiyama reagent (15), triethylamine were suspended in THF and amine (17) was added to mixture. The reaction mixture was refluxed for an optimal time. It was observed that aromatic amines having electron donating substituents like methyl and methoxy group furnished better yields of product as compared to aniline with electron withdrawing bromo substituents (Table 2, entries 1, 10, 2, 3 & 6, 7). On the other hand aromatic acid with electron donating as well as withdrawing groups showed excellent reactivity (Table 2, entries 5–9). However, 3,4,5-trimethoxybenzoic acid gave a lower yield even after longer reaction time (Table 2, entry 11). Aliphatic acid, propionic acid also furnished low yield of corresponding N-phenylpropionamide (Table 2, entries 13). The lower yield in case of 3,4,5-trimethoxybenzoic acid and propionic acid may be attributed to their lower reactivity towards first step of substitution. n-Butylamine also coupled with benzoic acid giving good yield of N-butylbenzamide (Table 2, entry 12). The heterocyclic amine, 2-aminobenzothiazole and 6-aminoquinoline also coupled with benzoic acid to give desired amide in moderate to good yield (48–59%) in 4–6 h using 15 as coupling reagent (Table 2, entry 14, 16). Nicotinic acid also coupled with aniline affording good yield of the desired product in 4 h. The longer reaction time taken by heteroaromatic acids and amines is attributed to lesser reactivity of acid and lower nucleophilicity of heteroamines. All the amides were obtained in excellent purity after simple workup without additional chromatography. The melting point and 1H NMR data for the synthesized amides were in agreement with the reported data in the literature. The by-product imidazolium salt-supported pyridone 20 was easily removed from the reaction mixture by simple washing. The structure of imidazolium salt-supported pyridone 20 was confirmed by 1H NMR, 13C NMR and IR spectroscopy (ESI†). It is worth to mention that recovered IL-supported pyridone (20) can be easily converted to reagent 15 by reacting with phosphorus oxychloride followed by anion metathesis with sodium trifluoromethanesulfonate.24a
| Entry | R | R′ | Product | Time (h) | Yielda (%) |
|---|---|---|---|---|---|
| a Isolated yield. | |||||
| 1 | C6H5 | C6H5 | 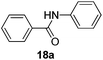 |
3 | 87 |
| 2 | C6H5 | 4-CH3C6H4 | 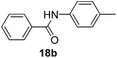 |
2 | 93 |
| 3 | C6H5 | 4-BrC6H4 | 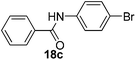 |
3 | 70 |
| 4 | C6H5 | C6H5CH2 | 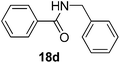 |
3 | 68 |
| 5 | 4-NO2C6H4 | C6H5 | 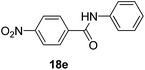 |
3 | 90 |
| 6 | 4-NO2C6H4 | 4-CH3C6H4 | 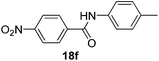 |
3 | 95 |
| 7 | 4-NO2C6H4 | 4-BrC6H4 | 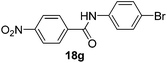 |
2 | 84 |
| 8 | 4-NO2C6H4 | C6H4CH2 | 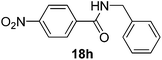 |
3 | 90 |
| 9 | 4-CH3OC6H4 | C6H5 | 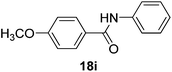 |
3 | 92 |
| 10 | C6H5 | 4-CH3OC6H4 | 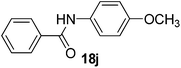 |
3 | 95 |
| 11 | 3,4,5-(CH3O)3C6H2 | 4-CH3C6H4 | 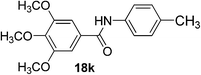 |
5 | 60 |
| 12 | C6H5 | C4H9 | 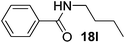 |
4 | 82 |
| 13 | C2H5 | C6H5 |  |
2.5 | 56 |
| 14 | C6H5 | C7H4NS |  |
4 | 48 |
| 15 | C5H4N | C6H5 |  |
4 | 63 |
| 16 | C6H5 | C9H6N |  |
6 | 59 |
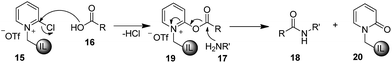
It is believed that the reaction proceeds through initial activation of carboxylic acid (16) by IL-supported 2-chloropyridinium triflate to give intermediate ester (19) which on subsequent reaction with amine (17) by nucleophilic attack furnishes amide (18) as product and IL-supported pyridone (20) as by-product (Scheme 2).
Experimental
General
The NMR (1H and 13C) spectra were recorded on 300 MHz and 400 MHz spectrometer in CDCl3 and DMSO-d6. The chemical shifts were expressed in ppm and coupling constants (J) in Hz. The IR spectra were recorded on ABB Bomen MB3000 FTIR spectrophotometer. The progress of the reaction was determined on thin-layer chromatography (TLC) performed on Merck-precoated silica gel 60-F254 plates. Melting points were determined on open capillary tube on MPA-120G EZ-Melt automated melting point apparatus and are uncorrected. 1,2-Dimethylimidazole, 2-chloropyridine, trifluoromethanesulfonic acid and other reagents and solvents were purchased from commercial sources and used without further purification unless otherwise specified.General procedure for the synthesis of (4-(3-chloropropoxy)phenyl)methanol (13)
4-Hydroxybenzaldehyde (8.00 g, 65 mmol), 1-bromo-3-chloropropane (6.46 mL, 65 mmol) and potassium carbonate (9.05 g, 65 mmol) were taken in dry acetone (25 mL) in a 250 mL round bottom flask. The reaction mixture was refluxed for 12 h. After completion of reaction, acetone was evaporated and residue was washed with water (20 mL) and extracted by ethyl acetate (15 mL × 2). The organic layer was dried with anhydrous sodium sulphate and evaporated under reduced pressure to get crude product. Crude product was purified by column chromatography on silica (60–120 mesh) using hexane and ethyl acetate as eluent (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) to get 7.86 g of pure 4-(3-chloropropoxy)benzaldehyde. It was further dissolved in dry THF (15 mL) at 0 °C, then sodium borohydride (2.24 g, 59 mmol) was added in pinches. After addition the resulting mixture was stirred at room temperature for 3 h. On completion of reaction, methanol was evaporated and water (15 mL) was added to the viscous residue. The solution was neutralized to pH 7 by 2 N HCl and extracted in ethyl acetate (15 mL × 2). The organic layer was washed with brine water and dried with anhydrous sodium sulfate and concentrated under reduced pressure to get pure product as white solid.
1, v/v) to get 7.86 g of pure 4-(3-chloropropoxy)benzaldehyde. It was further dissolved in dry THF (15 mL) at 0 °C, then sodium borohydride (2.24 g, 59 mmol) was added in pinches. After addition the resulting mixture was stirred at room temperature for 3 h. On completion of reaction, methanol was evaporated and water (15 mL) was added to the viscous residue. The solution was neutralized to pH 7 by 2 N HCl and extracted in ethyl acetate (15 mL × 2). The organic layer was washed with brine water and dried with anhydrous sodium sulfate and concentrated under reduced pressure to get pure product as white solid.
General procedure for the synthesis of imidazolium salt-supported benzyl alcohol (14)
A mixture of 13 (6.00 g, 30 mmol) and 1,2-dimethylimidazole (2.87 g, 30 mmol) was heated at 110 °C for 3 h to give thick viscous liquid. The viscous liquid was washed with ethyl acetate (3 × 20 mL) to remove unreacted starting materials to give pure chloride salt (8.56 g, 96%). Ion exchange of chloride was performed using (20 mL) aqueous potassium hexafluorophosphate (6.37 g, 35 mmol) solution at room temperature for 1 h. The resulting solid precipitate was filtered and washed with water and dried in vacuum to get pure 14.Yield: 11.37 g, 97%; mp: 115 °C; 1H NMR (300 MHz, DMSO) δ 7.65 (d, J = 2.0 Hz, 1H), 7.60 (d, J = 2.0 Hz, 1H), 7.23 (d, J = 8.5 Hz, 2H), 6.86 (d, J = 8.6 Hz, 2H), 5.05 (t, J = 5.6 Hz, 1H), 4.41 (d, J = 5.6 Hz, 2H), 4.29 (t, J = 6.8 Hz, 2H), 3.97 (t, J = 5.9 Hz, 2H), 3.73 (s, 3H), 2.55 (s, 3H), 2.19 (p, J = 6.4 Hz, 2H); 13C NMR (75 MHz, DMSO) δ 157.4, 144.9, 135.3, 128.4, 122.9, 121.4, 114.4, 64.5, 62.9, 45.3, 35.1, 29.1, 9.5.
General procedure for the synthesis of imidazolium salt-supported Mukaiyama reagent (15)
A mixture of 14 (11.00 g, 27 mmol) and 2-chloropyridine (14 g, 135 mmol) was suspended in 20 mL DCM at 0 °C under N2 atmosphere. Triflic anhydride (6.40 mL, 38 mmol) was added drop wise over 5 min. After complete addition, the resulting reaction mixture was allowed to stir at room temperature for 10 h. Reaction was monitored by TLC. On completion, the reaction mixture was filtered and washed with 20% DCM–methanol solution (20.0 mL) to furnish pure 15.Yield (9.68 g, 55%); mp 120 °C; 1H NMR (300 MHz, DMSO-d6) δ 9.27 (dd, J = 6.2, 1.3 Hz, 1H), 8.65 (td, J = 8.1, 1.5 Hz, 1H), 8.40 (dd, J = 8.2, 1.0 Hz, 1H), 8.21–8.14 (m, 1H), 7.65 (d, J = 2.0 Hz, 1H), 7.60 (d, J = 2.0 Hz, 1H), 7.39 (d, J = 8.7 Hz, 2H), 6.99 (d, J = 8.7 Hz, 2H), 5.90 (s, 2H), 4.28 (t, J = 6.9 Hz, 2H), 4.02 (t, J = 5.9 Hz, 2H), 3.73 (s, 3H), 2.55 (s, 3H), 2.20 (p, J = 6.3 Hz, 2H); 13C NMR (75 MHz, DMSO-d6) δ 159.2, 148.2, 148.1, 146.8, 144.9, 131.0, 130.8, 127.3, 124.8, 122.8, 121.4, 115.4, 64.7, 62.4, 45.2, 35.2, 29.0, 9.5.; ESI-MS (m/z): 502.4 [M-OTf]+ and 506.3 [M − PF6]+.
Representative procedure for the amide bond formation using 15
A mixture of 4-nitrobenzoic acid (0.030 g, 0.17 mmol) and imidazolium salt-supported Mukaiyama reagent 15 (0.1404 g, 0.21 mmol) was suspended in THF (5.0 mL) under nitrogen atmosphere. To this reaction mixture were added triethylamine (0.044 g, 0.43 mmol) and 4-bromoaniline (0.030 g, 0.17 mmol) in sequence. The resulting reaction mixture was allowed to reflux for 2 h. After completion of reaction, THF was evaporated to obtain viscous mixture. Product was extracted with 30% ethyl acetate–hexane (10 mL × 2) from viscous mixture and washed with 2 M HCl solution (5.0 mL × 3) and saturated bicarbonate solution (5.0 mL × 3) in order to remove any unreacted amine and by-products. The organic layer was washed with brine and dried over sodium sulphate and concentrated in vacuum to get pure amide 18g in 0.048 g (84%).Representative procedure for the regeneration of 15 from 20
A 10 mL clean oven dried round bottom flask was charged with IL-supported pyridone 20 (168 mg, 0.347 mmol) and phosphorus oxychloride (95 μL, 1.04 mmol) was added dropwise at room temperature. The reaction mixture was then heated at 80 °C for 8 h. After completion of the reaction, the volatile impurities were removed under reduced pressure. To the residue was added dry acetonitrile (5 mL) and sodium triflouromethansulfonate (80 mg). The reaction mixture was again refluxed for 12 h. After complete anion metathesis, the reaction mixture was filtered through a thin pad of silica to remove solid impurity and the filtrate was concentrated to get viscous product. The viscous liquid on washing with ethyl acetate (5 mL × 3) resulted into desired reagent 15.Conclusion
In conclusion, we have developed a shelf stable and efficient imidazolium salt-supported Mukaiyama reagent. This reagent is utilized for amide bond formation between carboxylic acids and amines. The significance of this protocol is evading column chromatography, shorter reaction time and good to excellent yields of amides. The isolated by-product imidazolium salt-supported pyridone 19 can be further utilized in regeneration of Mukaiyama reagent.24aAcknowledgements
The authors sincerely acknowledge the financial support from Council of Scientific and Industrial Research (CSIR), New Delhi (01(115)/13/EMR-II) for this research. KP thanks UGC, New Delhi for junior research fellowship (JRF) and MMK and SC thank CSIR, New Delhi for providing senior research fellowship (SRF).References
- (a) B. Rogge, Y. Itagaki, N. Fishkin, E. Levi, R. Rühl, S.-S. Yi, K. Nakanishi and U. Hammerling, J. Nat. Prod., 2005, 68, 1536–1540 CrossRef CAS PubMed; (b) S. D. Roughley and A. M. Jordan, J. Med. Chem., 2011, 54, 3451–3479 CrossRef CAS PubMed; (c) S. Ahmed, J. H. Mondal, N. Behera and D. Das, Langmuir, 2013, 29, 14274–14283 CrossRef CAS PubMed; (d) C. Yu and K. Mosbach, J. Org. Chem., 1997, 62, 4057–4064 CrossRef CAS.
- (a) D. Fattori, M. Porcelloni, P. D'Andrea, R.-M. Catalioto, A. Ettorre, S. Giuliani, E. Marastoni, S. Mauro, S. Meini, C. Rossi, M. Altamura and C. A. Maggi, J. Med. Chem., 2010, 53, 4148–4165 CrossRef CAS PubMed; (b) K. Lee, C. W. Park, W.-H. Jung, H. D. Park, S. H. Lee, K. H. Chung, S. K. Park, O. H. Kwon, M. Kang, D.-H. Park, S. K. Lee, E. E. Kim, S. K. Yoon and A. Kim, J. Med. Chem., 2003, 46, 3612–3622 CrossRef CAS PubMed.
- F. Damkaci and P. DeShong, J. Am. Chem. Soc., 2003, 125, 4408–4409 CrossRef CAS PubMed.
- M. P. Cassidy, J. Raushel and V. V. Fokin, Angew. Chem., Int. Ed., 2006, 45, 3154–3157 CrossRef CAS PubMed.
- T. Ribelin, C. E. Katz, D. G. English, S. Smith, A. K. Manukyan, V. W. Day, B. Neuenswander, J. L. Poutsma and J. Aubé, Angew. Chem., Int. Ed., 2008, 47, 6233–6235 CrossRef CAS PubMed.
- M. Hashimoto, Y. Obora, S. Sakaguchi and Y. Ishii, J. Org. Chem., 2008, 73, 2894–2897 CrossRef CAS PubMed.
- (a) T. M. U. Ton, C. Tejo, S. Tania, J. W. W. Chang and P. W. H. Chan, J. Org. Chem., 2011, 76, 4894–4904 CrossRef CAS PubMed; (b) V. Prasad, R. R. Kale, B. B. Mishra, D. Kumar and V. K. Tiwari, Org. Lett., 2012, 14, 2936–2939 CrossRef CAS PubMed; (c) S. Muthaiah, S. C. Ghosh, J.-E. Jee, C. Chen, J. Zhang and S. H. Hong, J. Org. Chem., 2010, 75, 3002–3006 CrossRef CAS PubMed; (d) S. C. Ghosh, J. S. Y. Ngiam, C. L. L. Chai, A. M. Seayad, T. T. Dang and A. Chen, Adv. Synth. Catal., 2012, 354, 1407–1412 CrossRef CAS.
- J. Xiao, G. Yuan, W. Huang, A. S. C. Chan and K. L. D. Lee, J. Org. Chem., 2000, 65, 5506–5513 CrossRef CAS PubMed.
- B. Zacharie, T. P. Connolly and C. L. Penney, J. Org. Chem., 1995, 60, 7072–7074 CrossRef CAS.
- R. M. Lanigan, P. Starkov and T. D. Sheppard, J. Org. Chem., 2013, 78, 4512–4523 CrossRef CAS PubMed.
- V. K. Das, R. R. Devi and A. J. Thakur, Appl. Catal., A, 2013, 456, 118–125 CrossRef CAS PubMed.
- (a) G. Quéléver, S. Burlet, C. Garino, N. Pietrancosta, Y. Laras and J.-L. Kraus, J. Comb. Chem., 2004, 6, 695–698 CrossRef PubMed; (b) C. A. G. N. Montalbetti and V. Falque, Tetrahedron, 2005, 61, 10827–10852 CrossRef CAS PubMed.
- T. Mukaiyama, M. Usui, E. Shimada and K. Saigo, Chem. Lett., 1975, 4, 1045–1048 CrossRef.
- E. Bald, K. Saigo and T. Mukaiyama, Chem. Lett., 1975, 4, 1163–1166 CrossRef.
- R. L. Funk, M. M. Abelman and K. M. Jellison, Synlett, 1989, 1989, 36–37 Search PubMed.
- T. Mukaiyama, M. Usui and K. Saigo, Chem. Lett., 1976, 5, 49–50 CrossRef.
- H. Huang, N. Iwasawa and T. Mukaiyama, Chem. Lett., 1984, 13, 1465–1466 CrossRef.
- H. L. Bradlow and C. A. Vanderwerf, J. Org. Chem., 1951, 16, 1143–1152 CrossRef CAS.
- P. Li and J.-C. Xu, Tetrahedron, 2000, 56, 8119–8131 CrossRef CAS.
- E. Convers, H. Tye and M. Whittaker, Tetrahedron Lett., 2004, 45, 3401–3404 CrossRef CAS PubMed.
- D. Donati, C. Morelli, A. Porcheddu and M. Taddei, J. Org. Chem., 2004, 69, 9316–9318 CrossRef CAS PubMed.
- S. Crosignani, J. Gonzalez and D. Swinnen, Org. Lett., 2004, 6, 4579–4582 CrossRef CAS PubMed.
- T. Nagashima, Y. Lu, M. J. Petro and W. Zhang, Tetrahedron Lett., 2005, 46, 6585–6588 CrossRef CAS PubMed.
- (a) Y. Sugiyama, Y. Kurata, Y. Kunda, A. Miyazaki, J. Matsui, S. Nakamura, H. Hamamoto, T. Shioiri and M. Matsugi, Tetrahedron, 2012, 68, 3885–3892 CrossRef CAS PubMed; (b) M. Matsugi, S. Nakamura, Y. Kunda, Y. Sugiyama and T. Shioiri, Tetrahedron Lett., 2010, 51, 133–135 CrossRef CAS PubMed.
- R. Berger, G. Resnati, P. Metrangolo, E. Weber and J. Hulliger, Chem. Soc. Rev., 2011, 40, 3496–3508 RSC.
- (a) N. V. Plechkova and K. R. Seddon, Chem. Soc. Rev., 2008, 37, 123–150 RSC; (b) M. Pucheault and M. Vaultier, in Ionic Liquids, ed. B. Kirchner, Springer, Berlin Heidelberg, 2010, vol. 290, pp. 83–126 Search PubMed.
- (a) M. K. Muthayala, B. S. Chhikara, K. Parang and A. Kumar, ACS Comb. Sci., 2011, 14, 60–65 CrossRef PubMed; (b) M. K. Muthayala and A. Kumar, ACS Comb. Sci., 2011, 14, 5–9 CrossRef PubMed; (c) M. K. Muthyala, S. Choudhary and A. Kumar, J. Org. Chem., 2012, 77, 8787–8791 CrossRef CAS PubMed; (d) M. Kumar Muthyala, S. Choudhary, K. Pandey, G. M. Shelke, M. Jha and A. Kumar, Eur. J. Org. Chem., 2014, 2014, 2365–2370 CrossRef CAS.
- J. Gao and G.-W. Wang, J. Org. Chem., 2008, 73, 2955–2958 CrossRef CAS PubMed.
- Z. Zhang, Y. Yu and L. S. Liebeskind, Org. Lett., 2008, 10, 3005–3008 CrossRef CAS PubMed.
- V. K. Haridasan, A. Ajayaghosh and V. N. R. Pillai, J. Org. Chem., 1987, 52, 2662–2665 CrossRef CAS.
- L. Kumar, T. Mahajan and D. D. Agarwal, Green Chem., 2011, 13, 2187–2196 RSC.
- C. L. Allen, S. Davulcu and J. M. J. Williams, Org. Lett., 2010, 12, 5096–5099 CrossRef CAS PubMed.
- A. R. Katritzky, C. Cai and S. K. Singh, J. Org. Chem., 2006, 71, 3375–3380 CrossRef CAS PubMed.
- Y. Wu, S. Wang, L. Zhang, G. Yang, X. Zhu, Z. Zhou, H. Zhu and S. Wu, Eur. J. Org. Chem., 2010, 2010, 326–332 CrossRef.
- M. A. Karim, W. H. Linnell and L. K. Sharp, J. Pharm. Pharmacol., 1960, 12, 82–86 Search PubMed.
- L.-F. Liu, H. Liu, H.-J. Pi, S. Yang, M. Yao, W. Du and W.-P. Deng, Synth. Commun., 2011, 41, 553–560 CrossRef CAS.
- D. Fajkusova and P. Pazdera, Synthesis, 2008, 2008, 1297–1305 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Copies of 1H and 13C NMR spectra for compounds 13, 14, 15, 18a–p and 19. See DOI: 10.1039/c4ra14856h |
| This journal is © The Royal Society of Chemistry 2015 |