Fabrication and electromagnetic wave absorption of novel Ni0.4Zn0.2Mn0.4Ce0.06Fe1.94O4–carbonyl iron composites
Abstract
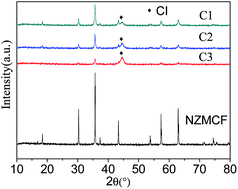
Ni0.4Zn0.2Mn0.4Ce0.06Fe1.94O4 (NZMCF)–carbonyl iron (CI) composites were fabricated using metal organic chemical vapor deposition (MOCVD) and were used as electromagnetic wave (EMW) absorbing materials. X-ray diffraction, scanning electron microscopy and energy dispersive spectrometry show that the NZMCF–CI composites were successfully prepared. Optimizing the weight ratio of CI would likely cause the composites to attain the EM parameters necessary in EMW absorbing materials. When the weight content of CI in the composites was approximately 27% (labeled as C2), the minimum reflection loss (RL) was −32.4 dB at 15.6 GHz and the corresponding thickness was 2.0 mm. The RL of C2 exceeds −10 dB from 6.6 to 18 GHz for an absorber thickness of 2.8 mm, which covers part of the C-band (3.8–8.2 GHz) and the entire X-band (8.2–12.4 GHz) and Ku-band (12.4–18 GHz).

 Please wait while we load your content...
Please wait while we load your content...