Simplified aptamer-based colorimetric method using unmodified gold nanoparticles for the detection of carcinoma embryonic antigen
Cheng Luo,
Wei Wen,
Fenge Lin,
Xiuhua Zhang,
Haoshuang Gu and
Shengfu Wang*
Hubei Collaborative Innovation Center for Advanced Organic Chemical Materials, Ministry of Education Key Laboratory for the Synthesis and Application of Organic Functional Molecules & College of Chemistry and Chemical Engineering, Hubei University, Wuhan 430062, PR China. E-mail: wangsf302@163.com; Fax: +86-27-88663043; Tel: +86-27-50865309
First published on 23rd December 2014
Abstract
Here, we described a simple and sensitive colorimetric method for the detection of carcinoma embryonic antigen (CEA), which was based on the phenomenon of salt-induced gold nanoparticles (AuNPs) aggregation and the conformation change of CEA's single-stranded DNA aptamer modified with a sulfhydryl group. Because of the reaction between the thiol group and AuNPs, the aptamer could bind to the surface of AuNPs strongly. AuNPs modified with aptamer had good stability in a high concentration of saline solution because of the repulsion of negative charges among the aptamers. In the presence of CEA, the aptamer tended to form a CEA-aptamer duplex instead of binding to the surface of AuNPs. As a result, the AuNPs changed its color from red to blue easily due to the salt-induced aggregation in the presence of CEA. Accordingly, the CEA was detected using this method. By adjusting the amount of the aptamer and NaCl added, a sensitive linear range for CEA was obtained. Under the optimum conditions, the detection limit for CEA was 3 ng mL−1. This rapid, label-free assay using colorimetric method showed high sensitivity for CEA detection, which made it a practical way in some cancer biomarker detections.
Introduction
CEA is an intracellular adhesion glycoprotein known as the tumor-associated antigens.1 Nowadays, CEA is usually used as tumor marker connected with several human cancers such as colorectal, gastric, pancreatic, and cervical.2–5 According to the updated research results, when the CEA level in the blood reaches to 20 ng mL−1, there is a big chance that the person suffers from cancer.6 Thus, a sensitive and precise method to detect the CEA is urgently needed.To diagnose the disease as early as possible, many methods have been used for the determination of CEA, including enzyme linked fluorescent assay,7 micropartical enzyme immunoassay,8 radioimmunoassays9,10 and chemiluminescence assays.11 Despite high specificity and sensitivity, these methods are expensive, time consuming and laborious, which may be critical limitations in some environments. In order to improve biomedical applications, developing a simple, fast, and sensitive method to detect CEA is desirable. In particular, such a simple yes/no research answer would be practical for diagnosis of the cancer in developing regions where healthcare facilities are relatively inferior.12
Therefore, colorimetric detection is a very attractive method because of its rapid visual assay on the spot without the need for any precise equipment.13 Recently, the use of the gold nanoparticles (AuNPs) has opened a new window for visual detection of many diseases. AuNPs are spheres with a typical diameter range from 2 nm to 50 nm.14 The surface plasmon absorption, which makes the AuNPs absorb and scatter more than those of conventional dyes, is responsible for their intense color change.15 For AuNPs, the color change from red to steel-blue was brought about by the transition of the colloidal AuNPs from dispersed to aggregated states, with the corresponding shift of surface plasmon absorption, which can be observed by naked eyes.16–18 These unique optical properties have allowed the application of AuNPs in simple and rapid colorimetric assays to offer more specificity and selectivity than the current methods. Moreover, their non-interference with other biomaterials makes them the most promising indicators for analytical and diagnostic studies.
Recently, aptamers have attracted considerable interest because of their competitive advantages compared with other biological tools.19 Aptamers are single-stranded oligonucleotides (DNA or RNA) that are selected by a process known as in vitro systematic evolution of ligands by exponential enrichment (SELEX).20 Moreover, they can bind to their targets with high affinity and specificity.21 It has been proved that using either unlabeled or labeled AuNPs as colorimetric reporters, unfolded single strand deoxyribonucleic acid (ssDNA) can be easily discriminated from the folded ssDNA or the double strand DNA (dsDNA).22,23 Hence, a variety of AuNPs-based colorimetric methods have been developed for the recognition and detection of target molecules, including protein,24 drugs,25 small molecules,26 inorganic ions27 and even cells. Compared with the aptamer-modified AuNPs-based methods that involve lengthy and complicated steps, such as modifying the aptamer onto the AuNPs and separating the modified AuNPs from the unmodified AuNPs or excess aptamer, the unmodified AuNPs-based colorimetric assays that require no modification or separation steps are more attractive.28
In this study, the method was based on the competing reaction of aptamer with the AuNPs and CEA. On the one hand, the aptamer with a thiol group at one end could bind to AuNPs, which increased the negative charge on the AuNPs colloid leading to the improvement of repulsion among the particles, preventing aggregation of AuNPs in a saline solution. On the other hand, the aptamer was short single-stranded nucleic acid oligomers, which could form a specific and firm three-dimensional structure to combine with CEA.29 Therefore, when AuNPs were added to a solution containing CEA and aptamer because of the combinative structure between CEA and aptamer, the aptamer was not free to bind to AuNPs to stabilize the AuNPs. Under this condition, AuNPs cannot be stabilized at high concentration of NaCl and aggregates, accompanied by the color change from red to blue; however, in the absence of CEA, the aptamer was free to bind to AuNPs and stabilize them, preventing their aggregation after adding NaCl, and thus the color remained red. The obvious distinction between the absence and presence of CEA made it rapid and visible to naked eye to detect CEA. Although ELISA based methods have recently become very popular, they require a stable source of antibodies. Moreover, preparation of antibody is laborious, expensive, and time-consuming. Compared with the ELISA, our method is one of the few that protein can be detected by simple “mix and detect” fashion, which greatly decreases the operating difficulty. It is also the first time that a AuNPs-based colorimetric method was adopted to detect CEA, which may be popular in the real application because it is simple, fast, and economical.
Moreover, this method may have application in diagnostic assay for other proteins or macromolecules by changing the aptamer, which provides another method in this field.
Materials and methods
2.1 Reagents and apparatus
Hydrogen tetrachloroaurate (III) trihydrate (HAuCl4·3H2O), trisodium citrate, CEA and tris(2-carboxyethyl) phosphine hydrochloride (TCEP) were purchased from Sigma-Aldrich (USA). All other reagents were of analytical grade. Oligonucleotides were synthesized and purified by Sangon (Shanghai, China). The base sequences of the oligonucleotides used in this work are listed as follows:aptamer: 3′-SH-ATACCAGCTTATTCAATT-5′ (ref. 30).
The solution containing CEA and aptamer was kept at 37 °C in the Biochemical Incubator PSH500A (Yinhe, Chongqing), then stirred on Magnetic Stirring Apparatus (Sile, Shanghai). Absorption spectra were recorded on a UV-3300 spectrophotometer (Japan) at a room temperature.
2.2 Preparation of gold nanoparticles
AuNPs were prepared according to a previously reported method.31,32 Briefly, 100 mL aqueous solution of hydrogen tetrachloroaurate trihydrate (1 mM) was heated to boiling with string in a round-bottom flask. Then, 10 mL of trisodium citrate (38.8 mM) was added into the solution rapidly and the solution was boiled for another 15 min with the color of the solution changing from dark blue to wine red. After that, heating the solution was stopped, but the solution was stirred until it cooled down to room temperature. Then, the prepared colloid AuNPs were stored in brown glass bottles at 4 °C. The final AuNPs had an average diameter of approximately 13 nm, which was characterized by Transmission Electron Microscopy (TEM), as shown in Fig. 2A.2.3 The preparation of aptamer solution
To get the aptamer solution, the aptamer was firstly dissolved in 0.1 M phosphate-buffered saline (PBS, pH = 7.4, hybridization buffer) to 10 μM solution. Then, 30 μL aptamer (10 μM) solutions and 15 μL TCEP (10 μM) were added into the samples containing different concentration that ranges from 0 to 120 ng CEA. Herein, TCEP is a reducing agent that is intended to disrupt any disulfide bonds (–S–S–) and ensure that the free –SH groups are ready to react with the gold surface. To have an adequate combination of CEA and aptamer, after stirring the mixed solution until blended well, they were put in the Biochemical Incubator PSH500A at 37 °C for 1 h.2.4 AuNPs-based colorimetric method
The previous mixed solution was added into 500 μL AuNPs solution separately and stirred in an appropriate speed for 1 h. Different amount of aptamer was bound to AuNPs to stabilize the AuNPs colloid. After the addition of NaCl into these solutions, these solutions displayed color gradient from red to blue. 100 μL of the solution was placed into a quartz cuvette, and then 200 μL of water was added before UV-vis measurement.Result and discussion
3.1 Overall strategy
In our system, the AuNPs in the solution were stabilized by adsorbed negative ions (citrate). Their repulsion prevented the strong van der Waals attraction between AuNPs from causing them to aggregate. However, high concentrations of salt will screen the charge on the surface of AuNPs, resulting in the aggregation of AuNPs. It has been reported that the aptamer with a thiol group at one end could bind to AuNPs,33 which can increase the negative charge on the AuNPs colloid, leading to a result that the repulsion among the particles was improved. The electrostatic repulsion prevented the strong van der Waals attraction and enhanced the stability of AuNPs against salt-induced aggregation, and thus the solution remained red under high-salt conditions. However, as depicted in Fig. 3, upon the addition of CEA, the conformation of CEA's aptamer changed from random coil structure to a specific and firm three-dimensional structure that combined with CEA. The three-dimensional structure prevented the exposure of the aptamer binding to AuNPs, and thus lost the ability of protecting the AuNPs under high-salt conditions. As a result, AuNPs aggregated under high-salt conditions and the color of AuNPs solution changed from red to blue. The overall strategy of this new colorimetric method is presented in Fig. 3. The first step involved the modification of the aptamer with the thiol group at one end and CEA. The aptamer formed a three-dimensional structure to combine with CEA. When mixed with AuNPs, the aptamer was not free to bind to the unmodified AuNPs; however, in the absence of CEA, the aptamer was free to bind to the surface of AuNPs as a result of the strong reaction of the thiol group and AuNPs. Importantly, the negative charges of the aptamer on the surface of AuNPs made it difficult for AuNPs to change color due to salt-induced nanoparticle aggregation. In contrast, AuNPs was easily observed for color change caused by aggregation upon the addition of NaCl in the absence of the thiolated aptamer.20 Moreover, if more aptamer grated on AuNPs surface, the barrier that prevented each particle from aggregation was bigger. Thus, the addition of appropriate amount of aptamer made the distinction more obvious with the presence and absence of CEA. Moreover, the quantity of NaCl added into the solution was also another important factor that influences the result. When the quantity of the aptamer and NaCl is controlled, the absorbance of the solution at 520 nm and 650 nm is in direct proportion to the quantity of CEA.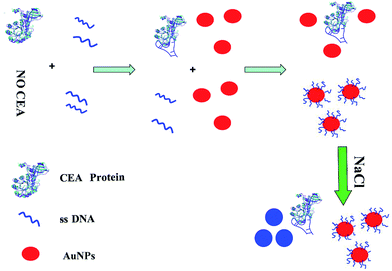 | ||
| Fig. 3 Schematic illustration of the colorimetric detection of CEA utilizing CEA's aptamer and unmodified AuNPs. | ||
3.2 The result of aptamer-modified AuNPs on detection of CEA
In order to confirm the assumption that the thiolated aptamer bound to AuNPs was the only reason for prevention of salt-induced aggregation, the difference of the AuNPs with the addition of aptamer in saline solution was observed. The AuNPs without the aptamer modified on the surface had an instantaneous color change from red to blue after the addition of NaCl, while the AuNPs solution containing the aptamer still remained red under identical conditions (Fig. 1 and 2). Absorption spectroscopic analysis showed a more obvious distinction in the presence of aptamer, which confirmed the well dispersed state of AuNPs in this solution. In contrast, a broad absorption spectrum band appeared, suggesting that the AuNPs were easily aggregated in a saline solution. These different results led to the fact that the aptamer binding to AuNPs enhanced the repulsion among AuNPs.To test the CEA detection strategy, the aptamer was first added into the sample containing CEA. Then, the solution was treated with AuNPs, followed by the addition of NaCl to observe the color change of AuNPs solution. The aptamer formed a three-dimensional structure29 to combine with CEA, which was stronger than the Au–S bond. Thus, the aptamer tended to combine with CEA in the presence of CEA, leading to a conclusion that the AuNPs were not modified with the aptamer and easily aggregated in a saline solution. In contrast, the aptamer was free to bind to AuNPs in the absence of CEA. The abundant negative charges of the aptamer enhanced the repulsion among AuNPs, consequently leading to the prevention of the salt-induced aggregation. As a result, the color of the AuNPs in the absence of CEA remained red after the addition of NaCl, while the color of AuNPs changed in the presence of CEA (Fig. 1 and 2). Therefore, a method based on the color change visible to the naked eye is used to detect CEA.
3.3 The effect of concentration of aptamer and NaCl
The amount of NaCl added to the AuNPs solution was the factor that influenced the aggregation of AuNPs. The color change of the solution was analyzed by measuring absorption spectrum at 520 nm and 650 nm. An appropriate NaCl concentration was beneficial to improve the sensitivity to detect CEA (Fig. 4A). After the addition of 30 μL of 10 μM aptamer, the data showed that 50 μL 0.5 M NaCl was the best choice to make the difference obvious between the presence and absence of CEA.Moreover, the concentration of aptamer was an important factor to the sensitivity. A high concentration of aptamer presented little difference in the presence of CEA, which was not sensitive to detect CEA. In contrast, a low concentration of aptamer was also not sensitive to detect CEA, because the AuNPs in the condition was easily aggregated with CEA or without CEA. Note that the effect of the difference in the concentration of the aptamer on AuNPs aggregation in a 40 ng mL−1 CEA concentration was evaluated (Fig. 4B). 5 μL, 10 μL, 20 μL, 30 μL and 40 μL of 10 μM aptamer were added into solutions containing 40 ng mL−1 CEA accompanied by 15 μL of 10 μM TCEP. After 1 h, these solutions were added to colloidal AuNPs solution separately and stirred for 1 h, followed by the addition of NaCl to induce aggregation of AuNPs. It was observed that increase in the concentration of aptamer led to an aggregation, showing a good stability in a high NaCl concentration. Thus, a proper concentration of aptamer in this colorimetric method was needed and we chose a 30 μL of 10 μM aptamer as the final condition.
3.4 Direct detection of CEA
To evaluate the sensitivity of CEA dependent colorimetric sensor and dynamic working range of the proposed system, the absorbance at 520 nm and 650 nm was monitored based on the above experiments. First, the CEA samples ranging from 10 ng mL−1 to 120 ng mL−1 were mixed with 30 μL of 10 μM aptamer and 15 μL of 10 μM TCEP. After 1 h, these solution were added to 500 mL AuNPs and stirred for 1 h. After the addition of 50 μL of 0.5 M NaCl to promote aggregation of AuNPs, these solutions were analysed with a spectrophotometer. As shown in Fig. 4C, the absorbance at 520 nm and 650 nm decreased with increase in the concentration of CEA. Linear relationship was found between the absorbance of A650/A520 and the concentration of CEA from 0 to 120 ng mL−1 with correlation coefficient of 0.998 (Fig. 4C); moreover, a detection limit of 3 ng mL−1 was obtained using the method of 3σ. Thus, a new colorimetric method of CEA was established. Here, it should be noted that the method is just a potential application and it still needs to be perfected for clinical diagnosis.3.5 Selection of the assay
In order to study the selectivity of the optical sensor, several commonly existing proteins were tested using the sensing system in identical condition, including myohemoglobin (MYO), mucoprotein (MUC) and bovine serum albumin (BSA). The amount of interfering proteins added to the sensing system was 200 ng. The sensing system treated with different proteins showed slight response from the absorptions at 520 nm and 650 nm and visual observation (Fig. 4D). Moreover, though the amount of CEA was lower than other proteins, the distinct color change in the presence of CEA could be easily observed by the naked eye. This colorimetric sensing system showed a good selection, which was suitable for convenient differentiation between CEA and other materials.3.6 Real sample analysis
To verify the practical application of the method, we tested spiked serum samples with different CEA concentrations. The real samples had been diluted 50 times before each experiment in order to diminish false positive contributions from the matrix. As shown in Table 1, the recoveries of the standard addition were between 94.4%, and 105.6%, which was a satisfactory result. These results reveal the potential application of this method for CEA detection in serum.Conclusions
In summary, this method was based on the competing reaction of aptamer with CEA and AuNPs. Using the electrostatic repulsion caused by a large number of negative charges of aptamer, which bound to the surface of AuNPs, the AuNPs were prevented from aggregating in a saline solution. Thus, the AuNPs solution still remained red in the absence of CEA, while the AuNPs solution changed from red to blue in the presence of CEA due to the combination of CEA and the aptamer. Thus, a new colorimetric method for the detection of CEA was developed. This is a quick and simple method that does not require special instrumentation, making it a practical application in some cancer biomarker detections. Moreover, the assay region, linear range and detection limit could be rationally tuned by varying the amount of aptamer and NaCl added to the sensing system. The tunable detection range and color change of the method were convenient and helpful in different detecting needs. Moreover, this method can be possibly extended for the detection of other proteins or nucleotides by changing the aptamer.Acknowledgements
This work was supported by the National Natural Science Foundation of China (no. 21175032) and the Natural Science Fund for Creative Research Groups of Hubei Province of China (no. 2014CFA015).References
- E. W. Martin Jr, W. E. Kibbey, L. Divecchia, G. Anderson, P. Catalano and J. P. Minton, Cancer, 1976, 37, 62–81 CrossRef CAS.
- J. E. Shively, V. Spayth, F. F. Chang, G. E. Metter, L. Klein, C. A. Presant and C. W. Todd, Cancer Res., 1982, 42, 2506–2513 CAS.
- A. Fuks, C. Banjo, J. Shuster, S. O. Freedman and P. Gold, Biochim. Biophys. Acta, 1975, 417, 123–152 CAS.
- C. G. Moertel, J. R. O’Fallon, V. L. W. Go, M. J. O'Connell and G. S. Thynne, Cancer, 1986, 58, 603–610 CrossRef CAS.
- M. Tatsuta, H. Yamamura, S. Noguchi, M. Ichii, H. Iishi and S. Okuda, Gut, 1984, 25, 1347–1351 CrossRef CAS PubMed.
- J. S. Cooper, M. D. Guo, A. Herskovic, J. S. Macdonald, J. A. Martenson Jr, R. Byhardt, A. H. Russell and J. J. Beitler, JAMA, J. Am. Med. Assoc., 1999, 281, 1623–1627 CrossRef CAS.
- J. L. Yuan, G. L. Wang, K. Majima and K. Matsumoto, Anal. Chem., 2001, 73, 1869–1876 CrossRef CAS.
- A. M. Delarosa and M. Kumakura, Anal. Chim. Acta, 1995, 312, 85–94 CrossRef CAS.
- J. M. MacSween, N. L. Warner, A. D. Bankhurst and I. R. Mackay, Cancer, 1972, 26, 356–360 CrossRef CAS.
- D. Tang, R. Yuan and Y. Chai, Anal. Chem., 2008, 80, 1582–1588 CrossRef CAS PubMed.
- W. Dungchai, W. Siangproh, J. M. Lin, O. Chailapakul, S. Lin and X. Ying, Anal. Bioanal. Chem., 2007, 387, 1965–1971 CrossRef CAS PubMed.
- D. Mabey, R. W. Peeling, A. Ustianowski and M. D. Perkins, Nat. Rev. Microbiol., 2004, 2, 231–240 CrossRef CAS PubMed.
- Y. K. Jung, T. W. Kim, J. Kim, J. M. Kim and H. G. Park, Adv. Funct. Mater., 2008, 18, 701–708 CrossRef CAS.
- J. Liu and Y. Lu, Nat. Protoc., 2006, 1, 246–252 CrossRef CAS PubMed.
- X. H. Huang, P. K. Jain, I. H. El-Sayed and M. A. El-Sayed, Nanomedicine, 2007, 2, 681–693 CrossRef CAS PubMed.
- R. Elghanian, J. J. Storhoff, R. C. Mucic, R. L. Letsinger and C. A. Mirkin, Science, 1997, 277, 1078–1081 CrossRef CAS.
- E. Katz and I. Willner, Angew. Chem., Int. Ed., 2004, 43, 6042–6108 CrossRef CAS PubMed.
- J. M. Nam, C. S. Thaxton and C. A. Mirkin, Science, 2003, 301, 1884–1886 CrossRef CAS PubMed.
- S. Song, L. W. Wang, J. Li, C. Fan and J. Zhan, TrAC, Trends Anal. Chem., 2008, 27, 108–117 CrossRef CAS PubMed.
- C. Tuerk and L. Gold, Science, 1990, 249, 505–510 CAS.
- T. Mairal, V. C. Özalp, P. L. Sánchez, M. Mir, I. Katakis and C. K. O'Sullivan, Anal. Bioanal. Chem., 2008, 390, 989–1007 CrossRef CAS PubMed.
- C. A. Mirkin, R. L. Letsinger, R. C. Mucic and J. J. Storhoff, Nature, 1996, 382, 607–609 CrossRef CAS PubMed.
- H. X. Li and L. Rothberg, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 14036–14039 CrossRef CAS PubMed.
- Y. Peng, L. D. Li, X. J. Mu and L. Guo, Sens. Actuators, B, 2013, 177, 818–825 CrossRef CAS PubMed.
- Y. F. Zhang, B. X. Li and X. L. Chen, Microchim. Acta, 2010, 168, 107–113 CrossRef CAS.
- Y. Y. Qi, L. Li and B. X. Li, Spectrochim. Acta, Part A, 2009, 74, 127–131 CrossRef PubMed.
- S. S. Zhan, M. L. Yu, J. Lv, L. M. Wang and P. Zhou, Aust. J. Chem., 2014, 67, 813–818 CrossRef CAS.
- L. Li, B. X. Li, Y. Y. Qi and Y. Jin, Anal. Bioanal. Chem., 2009, 393, 2051–2057 CrossRef CAS PubMed.
- Z. Y. Lin, G. Y. Zhang, W. Q. Yang, B. Qiu and G. N. Chen, Chem. Commun., 2012, 48, 9918–9920 RSC.
- D. Shangguan, Z. Cao, L. Meng, P. Mallikaratchy, K. Sefah, H. Wang, Y. Li and W. Tan, J. Proteome Res., 2008, 7, 2133–2139 CrossRef CAS PubMed.
- K. C. Grabar, R. Griffith, M. B. Hommer and M. J. Natan, Anal. Chem., 1995, 67, 735–743 CrossRef CAS.
- G. Frens, Nature physical science, 1973, 241, 20–22 CrossRef CAS.
- J. Wang, J. Li, A. J. Baca, J. Hu, F. Zhou, W. Yan and D. W. Pang, Anal. Chem., 2003, 75, 3941–3945 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |



