Aggregation, dissolution and cyclic regeneration of Ag nanoclusters based on pH-induced conformational changes of polyethyleneimine template in aqueous solutions†
Abstract
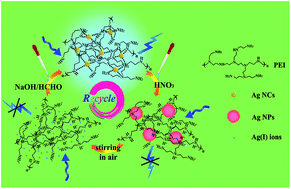
This paper reports a dramatic cyclic regeneration of polyethyleneimine-templated silver nanoclusters (PEI-AgNCs) based on the pH-induced conformational changes of polyethyleneimine (PEI) in aqueous solution. The PEI-AgNCs have been synthesized and found to be highly sensitive to the pH of the solution in air. The studies show that small AgNCs would gather to form larger silver nanoparticles (AgNPs) by adjusting the pH to 1.5 with a nitric acid solution. The AgNPs in solution was then transformed gradually to Ag(I) ions with stirring in air. Subsequently, the above Ag(I) ions were reduced again to AgNCs by changing the pH to about 9 with a NaOH solution and adding a certain amount of formaldehyde as a reductant to solution. The fluorescence and UV-visible absorption spectra recorded this process in detail. The transmission electron microscopy images, X-ray powder diffraction patterns, and Fourier transform infrared spectra further demonstrated that the cyclic transformation existed among AgNCs, AgNPs, and Ag(I) ions. The amino-rich PEI plays a crucial role in the regeneration of PEI-AgNCs. A large number of amino groups on PEI could be reversibly protonated by adjusting the pH of solution, leading to a change of the interaction between Ag and PEI, which has laid foundation for this work.

 Please wait while we load your content...
Please wait while we load your content...