Hierarchical flower-like Bi2WO6 hollow microspheres: facile synthesis and excellent catalytic performance†
Abstract
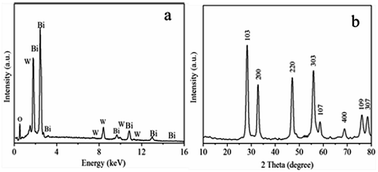
A one step method has been developed for the fabrication of hierarchical flower-like bismuth tungstate (Bi2WO6) hollow spheres via a solvothermal process. The size of these microspheres is about 1.5 μm, and the shells are composed of nanosheets with a thickness of about 15 nm. The product has a specific surface area of 95 m2 g−1. The formation mechanism of flower-like Bi2WO6 is proposed, which involves the nucleation and formation of nanoparticles followed by their self-assembly to microspheres, oriented growth, Ostwald ripening and transformation into flower-like hollow microspheres. The Bi2WO6 hollow spheres exhibit excellent visible light catalytic efficiency for the degradation of rhodamine B (RhB), up to 98% within 50 min. The efficiency remains at 92% after five photodegradation cycles due to the hierarchical hollow structure and large surface area.

 Please wait while we load your content...
Please wait while we load your content...