Luminescent Zn(II)–terpyridine metal–organic gel for visual recognition of anions†
Bowen Xiaoa,
Qiqi Zhanga,
Chengzhi Huangab and
Yuanfang Li*a
aKey Laboratory of Luminescent and Real-Time Analytical Chemistry (Southwest University), Ministry of Education, College of Chemistry and Chemical Engineering, Southwest University, Chongqing 400715, China. E-mail: liyf@swu.edu.cn; Fax: +86-23-68367257; Tel: +86-23-68254659
bCollege of Pharmaceutical Science, Southwest University, Chongqing 400716, China
First published on 3rd December 2014
Abstract
Luminescent metal–organic gels (MOGs), prepared with zinc sulfate and 4-[2,2′:6′,2′′-terpyridine]-4′-ylbenzoic acid (Hcptpy), have a good visual recognition ability for anions, which is visible with the naked eye, due to the affinity of anions to the zinc ion center in the MOGs.
Currently, supramolecular gels, especially metal–organic gels (MOGs), are a new class of multifunctional material, which have attracted great interest recently due to their desirable characteristics and porous gel structure.1 MOGs are generated by the self-assembly of metal complexes through noncovalent interactions, such as hydrogen bonding, π–π interaction, van der Waals, and ionic interactions.2 These metal-containing gels can react to a broader range of chemical and physical stimuli compared to other supramolecular gels, due to the flexible metal coordination and the complex noncovalent interactions.3 In terms of sensing chemistry, the responsiveness of MOGs is generally signaling through the change in color, due to spectral or phase transformation when disturbed by different kinds of triggers.4
Due to the introduction of novel properties of transition metals, such as catalytic and coordination behaviors, the smart MOGs have a variety of applications, including chiral recognition,3c,5 proton conduction,6 catalysis,7 adsorption8 and sensing.1c,9 Depending on the affinity of anions to the metal ion in the fluorophore–metal complexes,10 luminescent MOGs should be the excellent host receptors to recognize anionic species. Such a recognition event can be readily transformed into an external color or luminescence change once different anions have been incorporated into the MOGs.11 In comparison to other kinds of anion receptors, such as metal–organic frameworks (MOFs),12 nanoparticles,13 oligomers,14 and chelating organic molecules,10 the effective naked eye recognition of anions by stimuli-responsive luminescent MOGs is unprecedented.
The present paper describes a simple way to prepare luminescent MOGs comprised of zinc ion and 4-[2,2′:6′,2′′-terpyridine]-4′-ylbenzoic acid (Hcptpy) (Fig. 1a), which can effectively simultaneously recognize anions, including F−, Cl−, NO3−, Br−, I−, SCN−, N3−, PO43− and CO32−, visible with the naked eye. Upon heating an aqueous mixture of ZnSO4, Hcptpy and triethylamine (TEA) within 10 min, an opaque Zn(II)-gel formed, which shows blue fluorescence under the irradiation of 365 nm UV light (Fig. 1b). Gelation tests for the MOG formation showed that the gelation process depended on the reaction temperature and concentration of Zn2+, TEA and Hcptpy (Fig. S1–S4, ESI† concluded based on the tube inversion test). Only when the temperature was greater than 40 °C, the gel could be formed and the gel swelling degree increased with an increase in temperature (Fig. S1, ESI†). Additionally, differential scanning calorimetry (DSC) measurements showed that the as-prepared MOGs have a sol–gel transition temperature (Tgel) of 93 ± 3 °C.
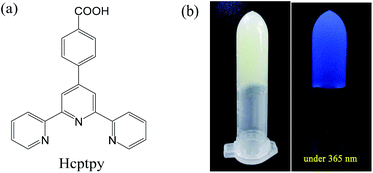 | ||
| Fig. 1 (a) The structure of Hcptpy. (b) Images (left: under day light; right: under 365 nm UV lamp) of the Zn(II)–Hcptpy gel. | ||
SEM and TEM measurements showed that all metal gelator molecules self-assembled into fine nanofibers with a diameter of ∼100 nm. Xerogels, which could be obtained by a vacuum freeze-drying method, were composed of nanoscale particles (Fig. 2c) and interconnected to form a porous gel matrix. Energy dispersive X-ray spectroscopy (EDS) analysis confirmed the presence of Zn, C, S, N, and O in the xerogels (Fig. S5, ESI†). The Fourier-transform infrared (FTIR) spectrum showed the characteristic vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O around 1700 cm−1 in Hcptpy, which disappeared after gel formation. The bands at ∼1612 cm−1 and ∼1390 cm−1, respectively, were the ν asym and ν sym (C
O around 1700 cm−1 in Hcptpy, which disappeared after gel formation. The bands at ∼1612 cm−1 and ∼1390 cm−1, respectively, were the ν asym and ν sym (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) of the carboxylate group in the xerogels (Fig. S6, ESI†). These results indicated that the carboxylate in Hcptpy was monodentate in the formation of the MOGs. A new peak at ∼1115 cm−1 in the xerogels was ascribed to the characteristic of SO42−.
O) of the carboxylate group in the xerogels (Fig. S6, ESI†). These results indicated that the carboxylate in Hcptpy was monodentate in the formation of the MOGs. A new peak at ∼1115 cm−1 in the xerogels was ascribed to the characteristic of SO42−.
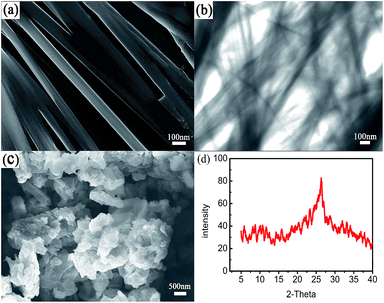 | ||
| Fig. 2 Characterization of the MOGs. (a) SEM image of the MOGs, (b) TEM image of the MOGs, (c) TEM image of the xerogels, and (d) X-ray diffraction (XRD) pattern of the xerogels. | ||
To gain further insight into the assembly process, XRD measurements were performed (Fig. 2d). Only one big diffraction peak at 2θ = 25.36 was observed for the metal–complex xerogels, which corresponded to a d spacing of 3.9 Å (based on Bragg’s equation). It resulted from the corresponding π stacking of the benzene ring in Hcptpy and the Zn⋯Zn interactions. A possible aggregation model to rationalize the self-organization of the MOGs is shown in Fig. 3. With the assistance of TEA, Hcptpy tried to link two Zn(II) ions through the tridentate chelating terpyridyl and monodentate carboxylate to form a one-dimensional chain.15 The fibers observed by SEM were formed by Zn(II)–Hcptpy coordination, π–π stacking and metal–metal interaction. These fibers were capable of trapping additional solvent further to form the stable MOGs. Moreover, both coordination interactions and the strong π–π stacking of the benzene rings played a key role in the formation of the MOGs.
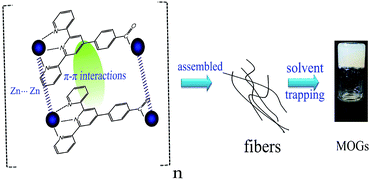 | ||
| Fig. 3 The assembly process of the MOGs by coordination, π–π stacking, metal–metal interaction and solvent trapping. | ||
The gel based fluorescent sensors have demonstrated the advantages of using the ordered, nanostructured gel surface for sensing rather than the solution.16 We investigated the applicability of our gel system for common anion sensing by simply adding 200 μL of the anion aqueous solution (NaF, KCl, KBr, KI, NaNO3, NaN3, KSCN, Na2CO3, and Na3PO4) to the MOGs. After standing at RT for 30 min, the MOGs undergo changes, which are clearly detectable with the naked eye (Fig. 4). Firstly, compared to the control (MOGs), only the color of the MOGs in I− changed into brown gradually under day light. A starch experiment for I−, after it was interacted with the MOGs, showed that there was no iodine generation. The MOGs collapsed and resulted in sol in CO32−, and precipitation in PO43− may be due to their greater ionic strength compared to other monovalent anions. Secondly, the fluorescence of the MOGs was enhanced when a Cl− or NO3− solution was added. The color of the others changed into grey, yellow and brown corresponding to the addition of Br−, SCN−, and N3− under 365 nm UV lamp light. However, the MOGs with the addition of F− scarcely changed. Therefore, we can visually distinguish I−, CO32− and PO43− with the naked eyes under daylight. Then, the simultaneous recognition of F−, Br−, SCN− and N3− was possible under the irradiation of the 365 nm UV lamp light according to their fluorescence changes (Fig. 4).
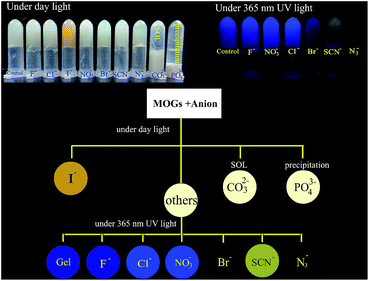 | ||
| Fig. 4 Anion recognition process visible to the naked eye. The inset photographs are the MOGs for anion sensing. The concentration of the anions is 0.03 M. | ||
We recorded the fluorescence spectral changes of the MOGs upon the addition of anions (Fig. 5). In the case of Cl− or NO3−, a significant enhancement of the fluorescence was found, while quenching occurred in the presence of Br−, N3−, I− and SCN−. Interestingly, a new peak at ∼550 nm after MOGs interacting with SCN− was observed. Moreover, there was no change observed in the case of F−. These observations are in agreement with the visual changes, which have already been shown in Fig. 4. The emission spectra of the MOGs at ∼450 nm in the presence of different concentrations of anions have been investigated and the detection limit has been listed in each plot (Fig. S7, ESI†). Additionally, the solid fluorescence absolute quantum yield of the xerogels (Φ = 0.013) was lower than that in the presence of Cl− (Φ = 0.028) or NO3− (Φ = 0.021) with excitation at 350 nm. Therefore, the MOGs are a useful colorimetric anion sensor for the effective detection of these target anionic species.
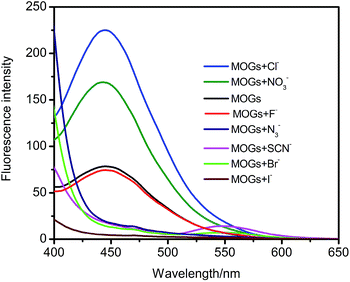 | ||
| Fig. 5 Fluorescence emission spectra of the MOGs in the absence and presence of different anions; λex = 350 nm; voltage = 400 V; the concentration of the anions is 0.03 M. | ||
Control experiments about the anion recognition process in the absence of Zn2+ have been studied (Fig. S8, ESI†). The fluorescence emission position of these mixture solutions in the presence of different anions was the same as the control, indicating that Zn2+ was the target site in the MOGs for the anion recognition. Based on the theory of Hard–Soft Acid–Base (HSAB theory),17 Cl−, NO3− or F−, which were the hard base, are prone to interact with the intermediate acid, Zn2+, in the MOGs by Coulomb force, which resulted in the fluorescence enhancement (Cl−, NO3−) or no significant change (F−). But for the soft base, I− or SCN−, a stronger covalent bond was formed between the anion and Zn2+, which led to a special colour or fluorescence change. In the case of N3− or Br−, which were the intermediate base, they would bind with Zn(II) by the force in-between the Coulomb force, and the covalent bond just quenched the fluorescence of the MOGs. Additionally, the interaction between the anions and the electron-deficient aromatic ring (anion–π interaction) and the hydrogen bond would also disturb the coordination of the MOGs, resulting in electron transfer or structural changes.18,19
In addition, we have investigated the XRD patterns of the MOGs after the interaction with different anions (Fig. 6). The big, wide diffraction peak at ∼25.36 of the MOGs was weakened or even disappeared instead of some other new diffraction peaks, which suggests that the structure of the MOGs has been transformed. All XRD patterns for the MOGs after interaction with an anion varied from one another, due to their different coordination modes, which lead to various internal structures as mentioned before. The SEM study showed that the two-dimensional fibrous MOGs have been destroyed and changed into other morphologies, such as nanoparticles and nanoplates (Fig. S9, ESI†). In combination with the XRD results, the high and clear diffraction peaks for the MOGs, with F−, Cl−, N3−, NO3− or CO32−, were attributed to the generation of a large number of particles or slices with uniform morphologies, while the messy and obscure diffraction peaks for the MOGs with Br−, I−, SCN− or PO43− were due to the growth of amorphous structures (Fig. S9, ESI†).
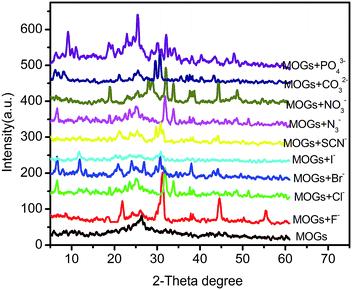 | ||
| Fig. 6 XRD patterns of the xerogels of the MOGs in the presence and absence of different anions. The concentration of the anions is 0.03 M. | ||
In conclusion, we have prepared fluorene-based Zn–Hcptpy MOGs with fiber-like networks by a simple process. The luminescent MOGs, with an anionic active affinity site, have been successfully explored for the simultaneous recognition of anions, including F−, Br−, I−, SCN−, N3−, CO32− and PO43−, with the naked eye. The recognition process has been monitored by fluorescence spectra, XRD and SEM. To our knowledge, such a colorimetric anion MOGs receptor, which is widely visible by the naked eye, is unprecedented.
Acknowledgements
We are grateful to the National Natural Science Foundation of China (NSFC, no. 21175109) and the special fund of Chongqing key laboratory (CSTC) for their financial support.Notes and references
- (a) B. J. Zhu, X. Y. Yu, Y. Jia, F. M. Peng, B. Sun, M. Y. Zhang, T. Luo, J. H. Liu and X. J. Huang, J. Phys. Chem. C, 2012, 116, 8601–8607 CrossRef CAS; (b) S. L. Xiang, L. Li, J. Y. Zhang, X. Tan, H. N. Cui, J. Y. Shi, Y. L. Hu, L. P. Chen, C. Y. Su and S. L. James, J. Mater. Chem., 2012, 22, 1862–1867 RSC; (c) J. H. Lee, S. Kang, J. Y. Lee, J. Jaworski and J. H. Jung, Chem.–Eur. J., 2013, 19, 16665–16671 CrossRef CAS PubMed; (d) S. Samai and K. Biradha, Chem. Mater., 2012, 24, 1165–1173 CrossRef CAS.
- (a) J. Y. Zhang and C. Y. Su, Coord. Chem. Rev., 2013, 257, 1373–1408 CrossRef CAS PubMed; (b) L. Li, S. Xiang, S. Cao, J. Zhang, G. Ouyang, L. Chen and C. Y. Su, Nat. Commun., 2013, 4, 1774 CrossRef PubMed; (c) S. Sengupta and R. Mondal, Chem.–Eur. J., 2013, 19, 5537–5541 CrossRef CAS PubMed.
- (a) W. W. Fang, X. Y. Liu, Z. W. Lu and T. Tu, Chem. Commun., 2014, 50, 3313–3316 RSC; (b) T. Tu, W. W. Fang, X. L. Bao, X. B. Li and K. H. Dötz, Angew. Chem., 2011, 50, 6601–6605 CrossRef CAS PubMed; (c) Q. X. Jin, L. Zhang, X. F. Zhu, P. F. Duan and M. H. Liu, Chem.–Eur. J., 2012, 18, 4916–4922 CrossRef CAS PubMed.
- (a) A. Dey, S. K. Mandal and K. Biradha, CrystEngComm, 2013, 15, 9769–9778 RSC; (b) K. Y. Liu, L. Y. Meng, S. L. Mo, M. M. Zhang, Y. Y. Mao, X. H. Cao, C. H. Huang and T. Yi, J. Mater. Chem., 2013, 1, 1753–1762 CAS; (c) S. C. Wei, M. Pan, K. Li, S. J. Wang, J. Y. Zhang and C. Y. Su, Adv. Mater., 2014, 26, 2072–2077 CrossRef CAS PubMed.
- (a) X. Chen, Z. Huang, S. Y. Chen, K. Li, X. Q. Yu and L. Pu, J. Am. Chem. Soc., 2010, 132, 7297–7299 CrossRef CAS PubMed; (b) W. G. Miao, L. Zhang, X. F. Wang, L. Qin and M. H. Liu, Langmuir, 2013, 29, 5435–5442 CrossRef CAS PubMed.
- S. Saha, E. M. Schön, C. Cativiela, D. Díaz Díaz and R. Banerjee, Chem.–Eur. J., 2013, 19, 9562–9568 CrossRef CAS PubMed.
- (a) Y. R. Liu, L. S. He, J. Y. Zhang, X. B. Wang and C. Y. Su, Chem. Mater., 2009, 21, 557–563 CrossRef CAS; (b) J. Y. Zhang, X. B. Wang, L. S. He, L. P. Chen, C. Y. Su and S. L. James, New J. Chem., 2009, 33, 1070–1075 RSC.
- Y. L. Hu, Y. F. Fan, Z. L. Huang, C. Y. Song and G. K. Li, Chem. Commun., 2012, 48, 3966–3968 RSC.
- (a) S. Barman, J. A. Garg, O. Blacque, K. Venkatesan and H. Berke, Chem. Commun., 2012, 48, 11127–11129 RSC; (b) C. L. Tan and Q. M. Wang, Inorg. Chem., 2011, 50, 2953–2956 CrossRef CAS PubMed; (c) J. S. Huo, Y. H. Zheng, S. T. Pang and Q. M. Wang, Cellulose, 2013, 20, 841–848 CrossRef CAS.
- S. M. Brombosz, A. J. Zucchero, R. L. Phillips, D. Vazquez, A. Wilson and U. H. F. Bunz, Org. Lett., 2007, 9, 4519–4522 CrossRef CAS PubMed.
- R. Martínez-Máñez and F. Sancenón, Chem. Rev., 2003, 103, 4419–4476 CrossRef PubMed.
- J. P. Ma, Y. Yu and Y. B. Dong, Chem. Commun., 2012, 48, 2946–2948 RSC.
- P. Rajakumar, R. Anandhan, D. Manoj and J. Santhanalakshmi, RSC Adv., 2014, 4, 4413–4419 RSC.
- R. Nishiyabu, M. A. Palacios, W. Dehaen and P. Anzenbacher Jr, J. Am. Chem. Soc., 2006, 128, 11496–11504 CrossRef CAS PubMed.
- J. Yang, R. X. Hu and M. B. Zhang, J. Solid State Chem., 2012, 196, 398–403 CrossRef CAS PubMed.
- (a) K. K. Kartha, S. S. Babu, S. Srinivasan and A. Ajayaghosh, J. Am. Chem. Soc., 2012, 134, 4834–4841 CrossRef CAS PubMed; (b) S. Bhowmik, B. N. Ghosh, V. Marjomaki and K. Rissanen, J. Am. Chem. Soc., 2014, 136, 5543–5546 CrossRef CAS PubMed.
- R. G. Pearson, J. Chem. Educ., 1968, 45, 581–648 CrossRef CAS.
- H. T. Chifotides and K. R. Dunbar, Acc. Chem. Res., 2013, 46, 894–906 CrossRef CAS PubMed.
- S. Dalapati, S. Jana and N. Guchhait, Spectrochim. Acta, Part A, 2014, 129, 499–508 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra14759f |
| This journal is © The Royal Society of Chemistry 2015 |
