Retracted Article: Multiwalled carbon nanotube wrapped nanoflake graphene composites for sensitive biosensing of leviteracetum
Abstract
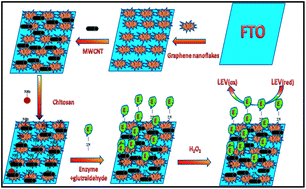
Current research work presents the detection of an antiepileptic drug i.e. leviteracetum by employing electrochemical impedance spectroscopy (EIS) using nanoflakes of graphene (GNF) and multiwalled carbon nanotube (MWCNT) decoratedfluorine-doped tin oxide (FTO) glass as sensing platform. This method also involved the covalent immobilized enzyme i.e. horse radish peroxidase (HRP) for LEV detection. Various stages of biosensor fabrication were characterized by TEM, XRD, SEM, EIS and CV. The sensing surface demonstrated a wide linear range (200 to 1000 μM) and a low detection limit 1 μM. The nano flakes-tubes composite based sensor showed good precision, analytical recovery and anti-interference ability which makes this sensing interface suitable for many pharmaceutical analyses. The applicability of the biosensor is to determine LEV levels in spiked serum samples.

 Please wait while we load your content...
Please wait while we load your content...