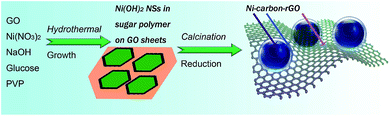
Anchoring superparamagnetic core–shells onto reduced graphene oxide: fabrication of Ni–carbon–rGO nanocomposite for effective adsorption and separation†
Hang Dua,
Zhen Wanga,
Yinghao Chena,
Yanyan Liua,
Yushan Liua,
Baojun Li*ab,
Xiangyu Wang*a and
Huaqiang Caob
aCollege of Chemistry and Molecular Engineering, Zhengzhou University, 100 Science Road, Zhengzhou 450001, P. R. China. E-mail: lbjfcl@zzu.edu.cn; xiangyuwang@zzu.edu.cn
bDepartment of Chemistry, Tsinghua University, 1 Tsinghua Park, Beijing 100084, P. R. China
First published on 2nd January 2015
Abstract
An adsorption technique based on nanocomposite materials is a simple and effective approach for the treatment of waste water. In this paper, a three-dimensional Ni–carbon–rGO (NGC) nanocomposite was synthesized through a hydrothermal process followed by carbonthermal reduction. The magnetic Ni nanoparticles (NPs) encapsulated in carbon shells were anchored onto reduced graphene oxide (rGO) sheets. This NGC was used as an adsorbent for rhodamine B (Rh-B) removal. Compared with other adsorbent materials, NGC exhibits higher adsorption rate and efficiency up to 4.4 L g−1 with good recyclability. The intrinsic superparamagnetism endows NGC enhanced separation efficiency with large adsorption capacity. The excellent removal ability for organic dyes makes NGC a useful candidate for wastewater treatment.
1. Introduction
Graphene has drawn much attention in the past ten years1–5 due to its unique two-dimensional (2D) honeycomb lattice structure,6 very high specific surface area (up to 2680 m2 g−1),7 superior mechanical rigidity,8 and excellent chemical stability.9 These superior physicochemical properties can meet theoretically the stringent requirements of high efficiency adsorbents.10,11 Graphene, graphene oxide (GO) and reduced graphene oxide (rGO) materials have also been used as an adsorbent for the removal of organic dyes or metal ions.10–12 It is of importance for the potential applications to employ the abundant surface functional groups of graphene-based composite materials for removal of pollutants. With the scarcity of fresh water resources, effective wastewater treatment technologies are aspired for recycling water resources more urgently than ever before.12–14 However, it is noteworthy that single graphene, GO and rGO as adsorbents have to suffer some problems, such as low adsorption capacities and separation inconveniences in the using process.10,12 Especially, the surface hydrophobicity of graphene and rGO makes it aggregate together in aqueous solution even under violent stirring and ultrasonication. High speed centrifugation or tediously filtration often is needed to separate the hydrophilic GO adsorbent from aqueous solution. The graphene (or rGO)–inorganic nanocomposites have been designed to solve the above problems.10,11,14 The existence of inorganic nano materials in the interlayer of graphene or rGO prevents graphene nanosheets from restacking in order to keep a high specific surface area and developed porous structure. The various surface functional groups on rGO also affect the crystallization course of inorganics in the preparation processes.10,11Generally, the wide applications of these high adsorption capacitance nanocomposites often were hampered by their low efficiency of separation and retrieve because of filtration or centrifugation after using.15,16 While superparamagnetic materials will find appropriate position in this field. Superparamagnetic material shows strong magnetic response to external field, which is vital for efficient separation. While no clear magnetic performance can be observed in the dispersing and adsorption processes, due to the small size of magnetic nanoparticles and superparamagnetic behaviour. The employing of magnetic components in composite adsorbent combines the separation convenience of magnetic matter and the high adsorption capacity of graphene–inorganic composites. It will provide an effective approach for the development of advanced adsorbent materials.
Recently, there are a few studies focused on the consolidation of graphene and inorganic materials to improve their physical behavior.17–21 The excellent properties of assembled three-dimensional (3D) architecture are attributed to the interactions between graphene and inorganic nanomaterials anchored on it. The fabrication of inorganic core–carbon shell structure22–24 is of benefit to improve the stability of nanoparticles (NPs) in composite materials. In graphene (or rGO)-based composite materials, the carbon shells wrapping on inorganic NPs will enhance the anchoring interaction between graphene or rGO and inorganic NPs and improve the structure stability of composite.25,26 Based on the above considerations, the novel graphene (rGO)-based 3D nanocomposites composed of core–shell structures with superparamagnetism are worth researching for the application in waste water treatment. The main idea is that the core–shell structure with superparamagnetic inorganic NPs as core and carbon layer as shells are fabricated, and then these core–shells will be anchored onto rGO to form a superparamagnetic 3D composite.
In this article, the magnetic Ni NPs encapsulated in carbon shells were anchored on the surface of rGO to form superparamagnetic 3D composite with mesoporous structure. Ni–carbon–rGO (NGC) nanocomposite showed excellent adsorption performances in waste water treatment. The enhanced adsorption capacity, recyclability and separation efficiency make this NGC a useful candidate for pollutant treatment. This superparamagnetic graphene-based 3D composite and its preparation strategy may find their application for the development of advanced functional materials.
2. Experimental section
2.1 Preparation of materials
Graphite oxide was synthesized using modified Hummer's method.10,27 Graphite oxide (200 mg) was dispersed into deionized water (200 mL) to form an aqueous suspension of GO under ultrasonic bath with agitating for 2 h. An aqueous solution (30 mL) of Ni(NO3)2·6H2O (8.905 g) and polyvinyl pyrrolidone (PVP, 0.150 g) was added to the aqueous GO suspension under stirring and then the mixture was stirred for further 4 h at room temperature. After that, an aqueous solution (20 mL) of NaOH (2.452 g) was added drop wise into the dispersion with agitate for another 30 min. Then glucose (2.252 g) was added, stirred for 30 min. The mixture was then transferred into a Teflon-lined stainless steel autoclave (500 mL), heated to 180 °C, and kept for 24 h. After cooled to room temperature, the solid was separated by filtration, washed with water and dried under 80 °C for 12 h. The solid was then heated to 550 °C with a rate of 10 °C min−1 and kept for 1 h in an N2 flow. After being cooled naturally to room temperature, the black powder (1.846 g) was obtained and nominated as NGC. The weight ratio of Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C
C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) rGO in NGC is = 36
rGO in NGC is = 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Two samples named 0.2Ni and 0.7C with weight ratio of Ni
1. Two samples named 0.2Ni and 0.7C with weight ratio of Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C
C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) rGO equal to 2
rGO equal to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7
7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2
1 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 were prepared by tuning the amount of rGO, glucose and Ni, respectively. Carbon content (weight percentage) in 0.2Ni and 0.7C were equal to each other with various weight ratio of C
5 were prepared by tuning the amount of rGO, glucose and Ni, respectively. Carbon content (weight percentage) in 0.2Ni and 0.7C were equal to each other with various weight ratio of C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) rGO. The carbon–rGO composite (C–rGO) was prepared by the similar procedure in the absence of Ni(NO3)2·6H2O, PVP and NaOH (see ESI†).
rGO. The carbon–rGO composite (C–rGO) was prepared by the similar procedure in the absence of Ni(NO3)2·6H2O, PVP and NaOH (see ESI†).
2.2 Characterization
The phase structure of as-prepared product was characterized with X-ray diffraction (XRD, Bruker D8 advance with Cu Kα, λ = 1.5418 Å) and a scanning rate of 1.2° min−1 in the 2 theta ranges from 5 to 80°. The average crystallite size of Ni NPs was estimated using the Scherrer formula: Dh = λ/(βh![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θh). Dh is the domain size of the diffraction line, λ is the wavelength of the Cu Kα source used, βh is the width in radians of the diffraction peak measured at half-maximum intensity (fwhm) corrected for instrumental broadening, and θh is the angle of the particular hkl reflection. The morphology of as-prepared products was studied by using high resolution transmission electron microscope (HRTEM, JEOL JEM-2010F electron microscope, operating at 200 kV). Fourier transform infrared (FT-IR) spectra were recorded on a Nicolet 380 spectrometer. Raman spectrum was recorded on a Renishaw RM-2000 with excitation from the 514 nm line of an Ar-ion laser with a power of about 5 mW. X-ray photoelectron spectrum (XPS) were recorded on a PHI quantera SXM spectrometer with an Al Kα = 280.00 eV excitation source, where binding energies were calibrated by referencing the C 1s peak (284.8 eV) to reduce the sample charge effect. The N2 sorption isotherms were measured on NOVA 1000e surface area and poresize analyzer (Quantachrome Instrument, USA) at 77 K. From the adsorption branch of isotherm curves in the P/P0 range between 0.05 and 0.35, the specific surface areas of NGC are calculated by Brunauer–Emmett–Tellet (BET) model. The pore size distribution was evaluated by the Barrett–Joyner–Halenda (BJH) model. The total pore volume was determined from the amount adsorbed at the relative pressure of about 0.99. Magnetic hysteresis loops were measured on Physical Property Measurement System (PPMS-9T) at 300 K with an applied magnetic field (Hmax = 50 kOe). The magnetization data also has been acquired in the so-called zero-field cooled modes. The sample is first cooled to low temperature in the absence of applied field. The magnetization data is recorded under an applied field of 500 Oe upon heating.
θh). Dh is the domain size of the diffraction line, λ is the wavelength of the Cu Kα source used, βh is the width in radians of the diffraction peak measured at half-maximum intensity (fwhm) corrected for instrumental broadening, and θh is the angle of the particular hkl reflection. The morphology of as-prepared products was studied by using high resolution transmission electron microscope (HRTEM, JEOL JEM-2010F electron microscope, operating at 200 kV). Fourier transform infrared (FT-IR) spectra were recorded on a Nicolet 380 spectrometer. Raman spectrum was recorded on a Renishaw RM-2000 with excitation from the 514 nm line of an Ar-ion laser with a power of about 5 mW. X-ray photoelectron spectrum (XPS) were recorded on a PHI quantera SXM spectrometer with an Al Kα = 280.00 eV excitation source, where binding energies were calibrated by referencing the C 1s peak (284.8 eV) to reduce the sample charge effect. The N2 sorption isotherms were measured on NOVA 1000e surface area and poresize analyzer (Quantachrome Instrument, USA) at 77 K. From the adsorption branch of isotherm curves in the P/P0 range between 0.05 and 0.35, the specific surface areas of NGC are calculated by Brunauer–Emmett–Tellet (BET) model. The pore size distribution was evaluated by the Barrett–Joyner–Halenda (BJH) model. The total pore volume was determined from the amount adsorbed at the relative pressure of about 0.99. Magnetic hysteresis loops were measured on Physical Property Measurement System (PPMS-9T) at 300 K with an applied magnetic field (Hmax = 50 kOe). The magnetization data also has been acquired in the so-called zero-field cooled modes. The sample is first cooled to low temperature in the absence of applied field. The magnetization data is recorded under an applied field of 500 Oe upon heating.
2.3 Adsorption test
Aqueous Rh-B solution (1.0 × 10−5 M) was used to evaluate the adsorption activity. The characteristic optical absorption peak of Rh-B at 544 nm was chosen to monitor the adsorption process. The adsorption separation was carried out according to the following procedure: adsorbent (50 mg) was added into aqueous Rh-B solution (220 mL) under stirring at ambient temperature. At given intervals (4 min), suspension (4 mL) was extracted and then centrifuged at 4000 rpm for 1 min to separate NGC from the supernatant. The static adsorption was carried out according to the following procedure: adsorbent (50 mg) was added into aqueous Rh-B solution (20 mL) without stirring at ambient temperature. At given intervals (10 min), suspension (4 mL) was extracted and then centrifuged at 4000 rpm for 1 min to separate the supernatant out. The visible spectroscopy (721, Shanghai Qinghua Tech. Ins. Lim.) equipped with a quartz cell of 1.0 cm path length was used to measure the absorbance. The other dye solution (1.0 × 10−5 M) including methylene blue, Congo red and methyl orange were used to investigate the adsorption performances of NGC for comparison. The adsorption capacity was calculated by following equation:| Qa = Va × Cb(Rh-B) × Wm/mNGC | (1) |
The wastewater treatment capacity was calculated by following equation:
| Vt = Va/mNGC | (2) |
3. Results and discussion
The designed NGC was composed of rGO sheets and Ni NPs capsulated in carbon shells (Scheme 1). Through precipitation reaction in the presence of GO and PVP, Ni(OH)2 nanosheets (NSs) were composited with GO sheets. In the following hydrothermal process, a glucose carbonization was performed to form sugar polymer wrapping on the Ni(OH)2 NSs (Fig. S2 and S3†). Calcinations' treatment assisted the sugar polymer to carbonize and reduce GO to rGO sheets. Meanwhile, Ni(OH)2 NSs also were reduced to Ni NPs by carbon element in the reactive sugar polymer. The sugar polymer was transformed to carbon, CO and CO2. This reduction process provided a Ni NPs@carbon core–shell structure composed of Ni NPs and carbon shells. The different density of Ni and Ni(OH)2 induced the shape and size change before and after reduction reaction. The Ni NPs@carbon shells were anchored on to rGO sheets to form a three-dimensional composite. The packing density (ρb) of NGC is 1.06 g cm−1 and the compacted density of NGC under 5.0 MPa is 2.42 g cm−1. The mass ratios of Ni, rGO, and carbon in NGC calculated from the TG analysis are 86.7, 5.4 and 7.9 wt%, respectively (Fig. S1, see ESI†).The morphology of as-prepared NGC was characterized by TEM images (Fig. 1a–d and S4, see ESI†). The morphology of NGC composite is almost consistent with that of r-GO sheets in the range of micrometers (Fig. 1a–d and S4a†). An amorphous carbon layer was observed clearly around the Ni NPs. These carbon layers formed carbon shells to protect Ni NPs against aggregation or from being oxidized by oxygen in air. The construction of carbon shells in the reduction process of Ni(OH)2 NSs and GO is the key originality of this preparation route. The Ni NPs@carbon shells anchored on the rGO sheets demonstrate irregular spherical with a mean diameter of 26 nm (Fig. 1e). The Ni NPs@carbon shells prevent rGO sheets from restacking thus to keep a high active surface area of NGC (Fig. 1a, b and S2b–d†). The Ni NPs encapsulated in carbon shells mainly anchored onto the surface of the rGO sheets (Fig. 1c and d). The selected area electron diffraction pattern provided the characteristic cycles corresponding to the (111), (200) and (220) planes of Ni and (002) plane of carbon, which exhibits the multi-crystalline nature of Ni NPs and carbon in NGC (Fig. 1f). Because of the capsulation with carbon shells, the crystalline lattice of Ni NPs cannot be observed (Fig. 1c and d). No alone Ni NPs, carbon shells or rGO is observed, which confirms the perfect combination between rGO and Ni NPs@carbon shells. The size of Ni NPs@carbon shells was about tens of nanometers.
 | ||
| Fig. 1 (a–d) TEM images of NGC, (e) particle size distribution of Ni NPs in NGC and (f) selected area electron diffraction pattern of NGC. | ||
The phase structure of NGC nanocomposite was investigated with XRD patterns. As shown in Fig. 2a, the diffraction peaks at 44.3°, 52.5° and 76.8° corresponding to the (111), (200) and (220) planes are the characteristic diffraction of crystal planes of face-centered cubic Ni (JCPDS card no. 65-2865). The mean crystalline size calculated for Ni NPs according to Scherrer formula with the diffraction peak of (111) plane is 26.5 nm, which is consistent with the HRTEM analysis. The broad peak centered at 2θ = 23.5° suggested the amorphous carbon structure.
 | ||
| Fig. 2 (a) XRD patterns of NGC and GO, (b) FT-IR patterns of NGC, C–rGO and GO, (c) Raman spectra of NGC, (d) XPS spectra of NGC, (e) C 1s and (f) Ni 2p spectra. | ||
The inconspicuous peak at 2θ = 26.2° corresponding to the (002) of carbon may be assigned to the somewhat graphitization of amorphous carbon catalyzed by the appearance of Ni NPs.28,29 The disappearance of the peaks at 11.5°, 26.6° and 42.4° shows that the reduction of GO is completed. Fig. 2b shows the FT-IR spectra of the as-synthesized NGC, C–rGO and GO. For NGC, the weak absorption band at 517 cm−1 should be assigned to the stretching vibration of Ni–O, which may be attributed to the reaction of Ni and oxygen-contained functional groups on the surface of rGO. The absorbance peaks at 1580 and 1620 cm−1 exhibited the existence of carbon and rGO.30,31 The absence of absorbance peak at 1724 cm−1 for carboxyl in NGC also indicates the reduction of GO to rGO.32,33 Fig. 2c shows the Raman spectra of the as-synthesized NGC, which display two prominent peaks. The G band at 1593 cm−1 is corresponding to the stretching modes of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C band of graphene domains, presenting an E2g mode of graphene. At 1348 cm−1 is the D band presenting the vibrations of carbon atoms dangling in disordered graphene.34 The stronger G peak (compared with the G peak from pristine graphene located in 1575 cm−1) was attributed to the residual of isolated double bonds after reduction, which echoes at frequencies higher than that of the G bands of the graphene.35,36 The decreased intensity ratio of D band to G band (ID/IG = 0.78) indicates the improvement in quality of rGO compared with GO (ID/IG = 0.83). This is very close to the previous report that the reduction of graphene is accompanied by the carbonization of glucose.37 The appearance of 2D peak around 2682 cm−1 indicates that the analyzed region is consisted of few layer graphene.38 The XPS survey spectra of NGC composite can certify the existence of Ni0 metal and the carbon of zero valence (Fig. 2d). The four different peaks centered at 284.8, 285.6, 286.8 and 289.0 eV are corresponding to carbon atoms in C–C, C–O, C
C band of graphene domains, presenting an E2g mode of graphene. At 1348 cm−1 is the D band presenting the vibrations of carbon atoms dangling in disordered graphene.34 The stronger G peak (compared with the G peak from pristine graphene located in 1575 cm−1) was attributed to the residual of isolated double bonds after reduction, which echoes at frequencies higher than that of the G bands of the graphene.35,36 The decreased intensity ratio of D band to G band (ID/IG = 0.78) indicates the improvement in quality of rGO compared with GO (ID/IG = 0.83). This is very close to the previous report that the reduction of graphene is accompanied by the carbonization of glucose.37 The appearance of 2D peak around 2682 cm−1 indicates that the analyzed region is consisted of few layer graphene.38 The XPS survey spectra of NGC composite can certify the existence of Ni0 metal and the carbon of zero valence (Fig. 2d). The four different peaks centered at 284.8, 285.6, 286.8 and 289.0 eV are corresponding to carbon atoms in C–C, C–O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and O
O and O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O groups, respectively (Fig. 2e).39,40 The intensities of carbon atom peaks corresponding to C–O, C
C–O groups, respectively (Fig. 2e).39,40 The intensities of carbon atom peaks corresponding to C–O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and O
O, and O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O are significantly weaker compared with that corresponding to C–C, confirming the high degree reduction of rGO.39,40 In the Ni 2p3/2 spectrum, the main peak at 852.8 eV and low-intensity broad peak at 870.2 eV are typical Ni0 metal spectra (Fig. 2f). The feeble peaks at 856.2 and 874.3 eV are the main peaks of Ni–OH, which would be attributed to the interaction between Ni0 metal nano-crystal and the hydroxyl on internal surface of carbon shells.41 From XPS spectrum, the atom ratio of Ni in NGC was calculated as 6.54% (equal to 23.4 wt%), which is lower than that calculated from TG analysis (86.7 wt%). This lower atom ratio of Ni should be attributed to the interference of carbon shells, which is also considered as another evidence of the construction of Ni NPs@carbon shells and composition of rGO sheets.
C–O are significantly weaker compared with that corresponding to C–C, confirming the high degree reduction of rGO.39,40 In the Ni 2p3/2 spectrum, the main peak at 852.8 eV and low-intensity broad peak at 870.2 eV are typical Ni0 metal spectra (Fig. 2f). The feeble peaks at 856.2 and 874.3 eV are the main peaks of Ni–OH, which would be attributed to the interaction between Ni0 metal nano-crystal and the hydroxyl on internal surface of carbon shells.41 From XPS spectrum, the atom ratio of Ni in NGC was calculated as 6.54% (equal to 23.4 wt%), which is lower than that calculated from TG analysis (86.7 wt%). This lower atom ratio of Ni should be attributed to the interference of carbon shells, which is also considered as another evidence of the construction of Ni NPs@carbon shells and composition of rGO sheets.
Specific surface area and porous structures of NGC were evaluated by nitrogen adsorption–desorption isotherm at 77 K (Fig. 3a). The isotherm of NGC exhibited the type of IV curve, which showed the existence of mesoporous structure. There is a hysteresis loop at relative pressure between 0.45 and 0.97, indicating that the pore diameter distribution of NGC is in the mesoporous region. The BET specific surface area and pore volume were 50.3 m2 g−1 and 0.16 cm3 g−1, respectively. These textural properties are comparable to those of typical adsorbent materials. The pore diameter distributions are narrow in the range from 15 to 25 nm with a most possible pore diameter of 19.3 nm, which should be caused by the uniform stacking of composite layers (Fig. 3b). The wide pore distribution around 47.0 nm should be attributed to the random stacking of composite layers (Fig. 3b). This developed porous structure provides ideal condition for high efficient adsorption. The decrease of surface area compared to rGO may be attributed to that the graphene layers coating on the surface of Ni NPs@carbon shells blocked some mesopores and macropores.
 | ||
| Fig. 3 (a) Nitrogen sorption isotherms and (b) corresponding pore diameter distribution for NGC. Insets in (b) are various stacking modes of composite layers in NGC. | ||
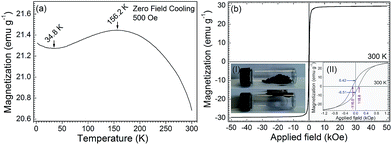
The magnetization performance of NGC was measured by the zero field cooling mode with an low-energy applied field of 500 Oe in the temperature range from 2 to 300 K (Fig. 4a). NGC exhibits ferromagnetic property under low temperature region (2–34.8 K) because of the ferromagnetic nature of Ni. Upon the Curie temperature (TC = 34.8 K), the ferromagnetic NGC gradually transformed to nearly super paramagnetic material due to the generally increasing thermodynamic fluctuation of Ni NPs. A maximum magnetization up to 21.45 emu g−1 appeared at 156.2 K.42 There is a slightly decreasing magnetization in the range from the blocking temperature (TB = 156.2 K) to room temperature (300 K), corresponding the paramagnetic property. At 300 K, a high magnetization of 20.68 emu g−1 still remained. The high and stable magnetization warrants that NGC is immediately magnetized by an applied field in a wide temperature range. The magnetization versus applied field (M–H curve) of NGC at 300 K confirmed the above analysis (Fig. 4b). The saturation magnetization (Ms) of NGC reached to 29.50 emu g−1 with an applied field larger than 6.5 kOe.43 NGC responded to the magnet very strongly and quickly (inset I in Fig. 4b). The hysteresis loop of NGC at 300 K showed that the very small coercivity (Hc) of 117.4 Oe are required to degauss NGC (inset II in Fig. 4b).44 The very low remanence magnetization (Mr) of 6.46 emu g−1 also is consistent with the small Hc. A small squareness (Sr = Mr/Hc) of 0.055 was obtained for NGC. These parameters are evidence for the super paramagnetic property of NGC.45 The superparamagnetic property and strong magnetic response endowed that NGC can be separated from liquid phase mixture system.
The success fabrication of 3D composite with superparamagnetic property encouraged us to investigate NGC in the adsorption removal of organic dyes from water. Fig. 5a shows the Rh-B adsorption removal performance of NGC, rGO, C–rGO, 0.2Ni and 0.7C in the time range of 0 to 10 min. Compared with rGO, C–rGO, 0.2Ni and 0.7C, NGC exhibits larger adsorption capacity and higher reaction rate which could be due to the neat structure composed of uniform carbon cover Ni particles anchored on the layer structure of graphene. The NGC composite adsorbed Rh-B quickly with large adsorption capacities. The adsorption capacity is attributed to the physical adsorption on rGO surface. Control experiments were carried out to distinguish the advantage of NGC. Compared with rGO and C–rGO (Fig. 5a), NGC showed better adsorption removal behavior with higher reaction rate and larger adsorption capacity up to 21.1 mg g−1 (equal to 23.0 mg cm−3 with packing density) in neutral environment (pH = 7.0). The wastewater treatment capacity of NGC reached to 4.4 L g−1 (pH = 7.0). Magnetic Ni NPs played a key role for the improved adsorption performances.10 Graphene or rGO cannot be well dispersed into aqueous solution because of its hydrophobic, so it possesses no substantial adsorption ability. Because Ni NPs prevent rGO from restacking, NGC can maintain a high specific surface area.27 The structure feature of Ni NPs helps NGC to be well dispersed into aqueous solution for effective adsorption.
The recyclability of NGC is of importance for its wide industrial application.13,46 The NGC (50 mg) recycling was conducted with ethanol (10 mL × 3) soaking for 10 min. Fig. 5b shows the recycling behavior of NGC in the adsorption removal of Rh-B from aqueous solution. Recycled NGC was obtained with NGC captured by a permanent magnet. And then Rh-B solution was added to start a new absorption separation cycles. Complete removal of Rh-B from water within 10 min shows a high adsorption separation efficiency. NGC almost retains adsorption ability and rate after being used and recycled for 7 times. Distilling of high concentration ethanol solution containing dye was conducted to recycle Rh-B from NGC. Colorless and transparent ethanol can be obtained from distillation. And the zero absorbance is the convincing proof that Rh-B could be effectively recycled by distillation process (Fig. S5†). The second pollution can be avoided effectively.
A magnet was used to absorb NGC from ethanol media. The solid was renewed after dried at 60 °C for 15 min. With recycled NGC to remove Rh-B from water, the completed adsorption was achieved. To investigate the kinetic behavior of NGC in adsorption, a larger amount of aqueous solution was used to decrease the adsorption speed. NGC (40 mg) was added into aqueous Rh-B solution (200 mL) under stirring at ambient temperature. Every interval of 2 min (this time was controlled strictly to seconds), suspension (4 mL) was extracted and measured the absorbance after centrifuged at 4000 rpm for 1 min. In acidic (pH = 3.6) environment, NGC also exhibited a superior adsorption separation performance comparable to that in neutral environment (Fig. 5c). In basic (pH = 11.1) environment, somewhat lower adsorption separation performance was obtained with slower adsorption speed. These pH-depending adsorption performance should be attributed to the various ionic strength of solution and charge state of dye molecules by pH change. Fig. 5d is the Rh-B adsorption removal performance of NGC in the time range from 2 to 10 min. It shows a typical linearity in the plot of ln(C/C0) versus time, presenting the occurred adsorption is a first-order reaction. This is in good agreement with the previous reports.13 Fig. 5e illustrates the static adsorption property of NGC, 0.2Ni and 0.7C in the time range from 0 to 60 min. The completed adsorption was achieved within 50 min without stirring. Powerful adsorption capacity of all the three samples have been observed, which affirm their effective utility in waste water treatment. The adsorption removal and magnetic separation of NGC are displayed in Fig. 5f–i. It was observed that the supernatant of mixture turned nearly colorless after being stirred for 10 min. The superparamagnetic property warrants that NGC can be dispersed homogeneously in the aqueous solution rather than attract each other to aggregate (Fig. 5e).47 The superparamagnetic NGC also is able to be easily separated from the aqueous solution in few seconds by placing a permanent magnet near the glass bottle (Fig. 5f and g). This effective separation process demonstrated that NGC could be used as a magnetic adsorbent to remove the organic pollutants from liquid phase.48 The thermal analysis curve of Rh-B in air atmosphere shown in Fig. S6† was used to investigate the degradation process. Rh-B is stable under 200 °C. The onset degradation temperature (T = 204.4 °C), which is defined as temperature at the 10.9 wt% weight loss, is obtained from the thermogravimetry curves. The final residual of 22.6 wt% at 610 °C should be identified as carbon remaining after the degradation.
In addition, the adsorption performance of NGC as adsorbent for various dye solution were tested (Fig. 6). The weaker adsorption performance of other dye solution but Rh-B were observed, which may be attributed to the mismatching of charge states between NGC and dye molecules. The small portion of adsorption performance that NGC retain for other dye solution could be explained as π–π interaction between graphene sheets in NGC composite and dye molecules.
4. Conclusions
In conclusion, a three-dimensional superparamagnetic NGC nanocomposite with high adsorption efficiency was prepared by a facile and simple approach. The morphological and structural characterizations confirmed that Ni NPs encapsulated in carbon shells were anchored uniformly onto rGO sheets. The adsorption removal of Rh-B from aqueous solution was conducted. The adsorption capacity of NGC for Rh-B reached up to 21.1 mg g−1. After being washed by ethanol, the magnetic recycling adsorbent still kept a high adsorption rate and adsorption efficiency. Compared with GO and rGO, NGC exhibits significantly improved adsorption ability and dispersivity. These features make NGC an outstanding candidate for application in environmental issues. The design and preparation method of this 3D superparamagnetic composite will be useful in the synthesis of other rGO-based functional materials. The extending research on composite materials including Fe, Co and Ru NPs is undergoing in this laboratory.Acknowledgements
Financial supports from the National Natural Science Foundation of China (no. U1204203, 21401168), the Outstanding Young Teacher Development Fund of Zhengzhou University (no. 1421316037) and the China Postdoctoral Science Foundation (no. 2013T60705) are acknowledged.Notes and references
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666 CrossRef CAS PubMed.
- A. K. Geim and K. S. Novoselov, Nat. Mater., 2007, 6, 183 CrossRef CAS PubMed.
- J. K. Wassei and R. B. Kaner, Acc. Chem. Res., 2013, 46, 2244 CrossRef CAS PubMed.
- V. Chabot, D. Higgins, A. P. Yu, X. C. Xiao, Z. W. Chen and J. J. Zhang, Energy Environ. Sci., 2014, 7, 1564 CAS.
- Z. Yan, Z. W. Peng and J. M. Tour, Acc. Chem. Res., 2014, 47, 1327 CrossRef CAS PubMed.
- J. M. Cai, P. Ruffieux, R. Jaafar, M. Bieri, T. Braun, S. Blankenburg, M. Muoth, A. P. Seitsonen, M. Saleh, X. L. Feng, K. Müllen and R. Fasel, Nature, 2010, 466, 470 CrossRef CAS PubMed.
- L. Britnell, R. V. Gorbachev, R. Jalil, B. D. Belle, F. Schedin, A. Mishchenko, T. Georgiou, M. I. Katsnelson, L. Eaves, S. V. Morozov, N. M. R. Peres, J. Leist, A. K. Geim, K. S. Novoselov and L. A. Ponomarenko, Science, 2012, 335, 947 CrossRef CAS PubMed.
- C. G. Lee, X. D. Wei, J. W. Kysar and J. Home, Science, 2008, 321, 385 CrossRef CAS PubMed.
- J. Malig, N. Jux and D. M. Guldi, Acc. Chem. Res., 2013, 46, 53 CrossRef CAS PubMed.
- B. J. Li and H. Q. Cao, J. Mater. Chem., 2011, 21, 3346 RSC.
- B. J. Li, H. Q. Cao, G. Yin, Y. X. Lu and J. F. Yin, J. Mater. Chem., 2011, 21, 10645 RSC.
- W. W. Li, H. Q. Yu and Z. He, Energy Environ. Sci., 2014, 7, 911 CAS.
- P. Sharma and M. R. Das, J. Chem. Eng. Data, 2013, 58, 151 CrossRef CAS.
- Y. S. Liu, B. J. Li, S. Zeng, X. D. Zhang, L. Fu, J. Ding, T. Z. Liu, X. S. Yan, Q. Cai and J. M. Zhang, J. Mater. Chem. A, 2014, 2, 4264 CAS.
- C. J. Madadrang, H. Y. Kim, G. H. Gao, N. Wang, J. Zhu, H. Feng, M. Gorring, M. L. Kasner and S. F. Hou, ACS Appl. Mater. Interfaces, 2012, 4, 1186 CAS.
- S. Linley, Y. Y. Lin, C. J. Ptacek, D. W. Blowes and F. X. Gu, ACS Appl. Mater. Interfaces, 2014, 6, 4658 CAS.
- S. B. Yang, X. L. Feng, L. Wang, K. Tang, J. Maier and K. Müllen, Angew. Chem., Int. Ed., 2010, 49, 4795 CrossRef CAS PubMed.
- H. L. Wang, Y. Yang, Y. Y. Liang, G. Y. Zheng, Y. G. Li, Y. Cui and H. J. Dai, Energy Environ. Sci., 2012, 5, 7931 CAS.
- B. J. Li, H. Q. Cao and G. Yin, J. Mater. Chem., 2011, 21, 13765 RSC.
- Y. Y. Liang, H. L. Wang, H. S. Casalongue, Z. Chen and H. J. Dai, Nano Res., 2010, 3, 701 CrossRef CAS PubMed.
- G. M. Zhou, D. W. Wang, F. Li, L. L. Zhang, N. Li, Z. S. Wu, L. Wen, G. Q. Lu and H. M. Cheng, Chem. Mater., 2010, 22, 5306 CrossRef CAS.
- Y. M. Ren, J. Zhang, Y. Y. Liu, H. B. Li, H. J. Wei, B. J. Li and X. Y. Wang, ACS Appl. Mater. Interfaces, 2012, 4, 4776 CAS.
- D. J. Xue, S. Xin, Y. Yan, K. C. Jiang, Y. X. Yin, Y. G. Guo and L. J. Wan, J. Am. Chem. Soc., 2012, 134, 2512 CrossRef CAS PubMed.
- S. G. Chen, Z. D. Wei, X. Q. Qi, L. C. Dong, Y. G. Guo, L. J. Wan, Z. G. Shao and L. Li, J. Am. Chem. Soc., 2012, 134, 13252 CrossRef CAS PubMed.
- H. Guo, R. Mao, D. X. Tian, W. Wang, D. P. Zhao, X. J. Yang and S. X. Wang, J. Mater. Chem. A, 2013, 1, 3652 CAS.
- H. Kim, Y. Son, C. Park, J. Cho and H. C. Choi, Angew. Chem., Int. Ed., 2013, 52, 5997 CrossRef CAS PubMed.
- B. J. Li, H. Q. Cao, J. Shao, G. Q. Li, M. Z. Qu and G. Yin, Inorg. Chem., 2011, 50, 1628 CrossRef CAS PubMed.
- X. C. Gui, J. Q. Wei, K. L. Wang, A. Y. Cao, H. W. Zhu, Y. Jia, Q. K. Shu and D. H. Wu, Adv. Mater., 2010, 22, 617 CrossRef CAS PubMed.
- Y. Ito, Y. Tanabe, H. J. Qiu, K. Sugawara, S. Heguri, N. H. Tu, K. K. Huynh, T. Fujita, T. Takahashi, K. Tanigaki and M. W. Chen, Angew. Chem., Int. Ed., 2014, 53, 4822 CrossRef CAS PubMed.
- S. Y. Wang, D. S. Yu, L. M. Dai, D. W. Chang and J. B. Baek, ACS Nano, 2011, 5, 6202 CrossRef CAS PubMed.
- P. M. Sudeep, T. N. Narayanan, A. Ganesan, M. M. Shaijumon, H. Yang, S. Ozden, P. K. Patra, M. Pasquali, R. Vajtai, S. Ganguli, A. K. Roy, M. R. Anantharaman and P. M. Ajayan, ACS Nano, 2013, 7, 7034 CrossRef CAS PubMed.
- N. A. Kumar, H. J. Choi, Y. R. Shin, D. W. Chang, L. M. Dai and J. B. Baek, ACS Nano, 2012, 6, 1715 CrossRef CAS PubMed.
- X. L. Wu, T. Wen, H. L. Guo, S. B. Yang, X. K. Wang and A. W. Xu, ACS Nano, 2013, 7, 3589 CrossRef CAS PubMed.
- D. C. Geng, B. Wu, Y. L. Guo, B. R. Luo, Y. Z. Xue, J. Y. Chen, G. Yu and Y. Q. Liu, J. Am. Chem. Soc., 2013, 135, 6431 CrossRef CAS PubMed.
- H. L. Wang, J. T. Robinson, X. L. Li and H. J. Dai, J. Am. Chem. Soc., 2009, 131, 9910 CrossRef CAS PubMed.
- W. S. Leong, H. Gong and J. T. L. Thong, ACS Nano, 2014, 8, 994 CrossRef CAS PubMed.
- B. J. Li, H. Q. Cao, J. Shao and M. Z. Qu, Chem. Commun., 2011, 47, 10374 RSC.
- A. Dimiev, D. V. Kosynkin, A. Sinitskii, A. Slesarev, Z. Z. Sun and J. M. Tour, Science, 2011, 331, 1168 CrossRef CAS PubMed.
- L. Y. Zhao, R. He, K. T. Rim, T. Schiros, K. S. Kim, H. Zhou, C. Gutiérrez, S. P. Chockalingam, C. J. Arguello, L. Pálová, D. Nordlund, M. S. Hybertsen, D. R. Reichman, T. F. Heinz, P. Kim, A. Pinczuk, G. W. Flynn and A. N. Pasupathy, Science, 2011, 333, 999 CrossRef CAS PubMed.
- W. H. Lin, T. H. Chen, J. K. Chang, J. I. Taur, Y. Y. Lo, W. L. Lee, C. S. Chang, W. B. Su and C. I. Wu, ACS Nano, 2014, 8, 1784 CrossRef CAS PubMed.
- D. P. Dubal, G. S. Gund, C. D. Lokhande and R. Holze, ACS Appl. Mater. Interfaces, 2013, 5, 2446 CAS.
- L. Hofstetter, A. Geresdi, M. Aagesen, J. Nygård, C. Schönenberger and S. Csonka, Phys. Rev. Lett., 2010, 104, 246804 CrossRef CAS.
- M. Han, Q. Liu, J. H. He, Y. Song, Z. Xu and J. M. Zhu, Adv. Mater., 2007, 19, 1096 CrossRef CAS.
- S. Carenco, C. Boissière, L. Nicole, C. Sanchez, P. L. Floch and N. Mézailles, Chem. Mater., 2010, 22, 1340 CrossRef CAS.
- C. C. Yeh and D. H. Chen, Appl. Catal., B, 2014, 150, 298 CrossRef PubMed.
- H. B. Li, X. C. Gui, L. H. Zhang, S. S. Wang, C. Y. Ji, J. Q. Wei, K. L. Wang, H. W. Zhu, D. H. Wu and A. Y. Cao, Chem. Commun., 2010, 46, 7966 RSC.
- J. Guo, W. L. Yang and C. C. Wang, Adv. Mater., 2013, 25, 5196 CrossRef CAS PubMed.
- B. J. Li, H. Q. Cao, J. F. Yin, Y. M. A. Wu and J. H. Warner, J. Mater. Chem., 2012, 22, 1876 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Thermal analysis curves and HRTEM images of NGC, TEM images and XRD pattern of intermediate, photo images of the recycling Rh-B and ethanol, and thermal analysis of Rh-B. See DOI: 10.1039/c4ra14651d |
| This journal is © The Royal Society of Chemistry 2015 |