Synthesis and investigation of water-soluble anticoagulant warfarin/ferulic acid grafted rare earth oxide nanoparticle materials†
Mei-Ling Yangab and
Yu-Min Song*a
aCollege of Chemistry and Chemical Engineering, Northwest Normal University, Lanzhou 730070, China. E-mail: songym@nwnu.edu.cn; Tel: +86-931-7972509
bDepartment of Applied Chemistry, Yuncheng University, Yuncheng 044000, China
First published on 9th January 2015
Abstract
To overcome the poor solubility of anticoagulant nano-rare earth (RE) oxides and warfarin/ferulic acid in water, two series of water-soluble anticoagulant materials, RE2O3-TDI-PEG-warfarin and RE2O3-TDI-PEG-ferulic acid (TDI = toluene 2,4-diisocyanate, RE = La, Eu, Nd, Sc, Sm, Dy), were prepared via a grafting method. The novel materials were characterized by IR, TG, XRD, SEM, TEM, 1H and 13C NRM and particle size tests. The results confirmed that warfarin and ferulic acid were successfully modified onto the surfaces of the rare earth nano-oxides. The anticoagulant properties were evaluated on the basis of coagulation time (CT), recalcification time (RT), activated partial thromboplastin time (APTT) and prothrombin time (PT). It was demonstrated that the novel hybrid materials have better cell compatibility and anticoagulant action than that of warfarin sodium or ferulic acid, due to the increased solubility (>10 mg mL−1) in water and synergy between nano-RE2O3 and warfarin/ferulic acid. It was also found that the anticoagulant time of the hybrid materials depends on the concentration i.e., the higher the concentration of hybrid materials, the longer the anticoagulant time. Additionally, Sc2O3-TDI-PEG-warfarin and Sc2O3-TDI-PEG-ferulic acid exhibited the best anticoagulant properties, which indicate potential application in the medicinal area. These results also provide the opportunity for the development of anticoagulant complexes and the potential for application in other related fields.
1. Introduction
Warfarin (W) and ferulic acid (FA) have long been the major drugs for anticoagulation in clinical applications1–3 due to their advantages of oral availability, convenient application, inexpensive price, and lasting effect (Scheme 1). However, Shouming Wang4 pointed out that there are a few drawbacks from the extended use of warfarin compounds as anticoagulant drugs, such as spontaneous bleeding and skeletal development retardation. Meanwhile, FA is widely studied in combination therapies in treating cancer, myocardial infarction, atherosclerosis,5 acute cerebral infarction6 and for kidney protection.7 However, the application of FA in clinics is limited by its difficulty in penetrating the biological membrane bilayer, and the poor solubility in water. Therefore, it is important to find a substitute that has lower toxicity, better anticoagulant properties and fewer side effects compared to warfarin/ferulic acid and their derivatives. One useful strategy to improve the efficiency of warfarin and ferulic acid is to fabricate hybrid materials by introducing new components. Studies on the properties of warfarin complexes composited with rare earth complexes8 or transition metals9 by the groups of Jiao and Dong have demonstrated that these complexes exhibit better hemorrhagic anticoagulant properties, lower toxicity and high stability. Meanwhile, complexes of ferulic acid with transition metals or rare earth ions were found to prolong the anticoagulant time and recalcification time.10 However, the poor water solubility still limits their applications in the clinical sense.Rare earth complexes including rare earth nano-oxides as anticoagulants have been broadly investigated.11,12 For example, recently, Wang and co-workers13 presented the anticoagulant performance of heparin/rare earth nano-oxide hybrids. Ni and Zhou14,15 et al. reported that the rare earth elements can replace Ca2+ to inhibit the coagulation of blood.
Though the rare earth nano-oxide ferulic acid and warfarin complexes have a significant anticoagulant effect, they suffer from poor water solubility along with the problem of spontaneous bleeding due to large amounts of drug usage. As a result, it is necessary to develop novel anticoagulant materials with high water solubility and only a small amount of drug usage. On this basis, herein two series of water soluble RE2O3-TDI-PEG-Warfarin and RE2O3-TDI-PEG-Ferulic acid complexes were prepared (Fig. 1) by combining water soluble H2N–PEG–NH2 with RE2O3 nano-particles and ferulic acid/warfarin. These novel hybrid materials were characterized by infrared spectroscopy (IR), thermo-gravimetric analysis (TG), scanning electron microscopy (SEM), powder X-ray diffractometry (powder-XRD), transmission electron microscopy (TEM), 1H and 13C NMR spectroscopy and particle size analysis. The anticoagulant properties were evaluated on the basis of anticoagulant time (CT), recalcification time (RT), activated partial thromboplastin time (APTT) and prothrombin time (PT). The results demonstrated that the novel hybrid materials have longer anticoagulation times than that of ferulic acid or warfarin, and significantly improved water solubility (>10 mg mL−1) compared with bare ferulic acid (<1 mg mL−1) and warfarin (<1 mg mL−1). In addition, the cell adhesion of the materials remained the same as untreated control samples.
2. Experimental
2.1 Materials and measurement
RE2O3 nano-oxides were purchased from the Shanghai Yuelong Plant (China). Warfarin sodium was acquired from the Shanghai Sixteenth Pharmaceutical factory (China) (m.p. 200–205 °C), and was treated with HCl to produce warfarin (m.p. 160.5–161.0 °C, literature:8 159.5–160.5 °C). Ferulic acid and PEG-4000 were obtained from the Shanghai Zhongqin Chemical Reagent Limited Company (China). The H2N–PEG–NH2 was prepared according to the literature method.16 Toluene 2,4-diisocyanate (TDI) was acquired from the Shanghai Kangda Amino acid factory (China). All other chemicals and solvents were of analytical grade.FT-IR spectra of the materials were obtained on a Nicolet NEXUS 670 FT-IR spectrometer with a wave number range of 4000–400 cm−1 from KBr pellets. Thermogravimetric analysis (TG) was performed through a Perkin-Elmer TGA7 instrument in flowing N2 and a heating rate at 10° min−1 in the 30–700° range. The morphological features of all materials were identified through scanning electron microscopy (SEM) (XL-20 (Philips, Netherlands)). Transmission electron microscope (TEM) was used to determine the morphology of the hybrid materials. Powder X-ray diffraction (powder-XRD) analysis was obtained with a Philips XPert Pro diffractometer using Ni-filtered and Cu Kα radiation. Particle size analysis was performed on a Zetasizer Nano-Zs laser particle size instrument (Malvern, UK). 1H and 13C NMR spectra were recorded using a Bruker 400 MHz and d6-DMSO as a solvent.
2.2 Modification of the surfaces of rare earth nano-oxides
The rare earth nano-oxides (RE2O3) were dried in a vacuum oven at 120 °C for 7–8 h before use. RE2O3 (5 g), TDI (5 g) and 100 mL anhydrous acetone were added into a flask and sonicated under N2 atmosphere for 2 h. To this solution, an appropriate amount of dibutyltin dilaurate was added and the solution was sonicated at 80 °C for 2 h. Finally, the reaction mixture was separated by filtration and the obtained solid was washed with anhydrous acetone (30 mL). The resulting materials were then heated in a vacuum oven at 80 °C. The obtained solid was labeled as RE2O3-TDI and was slightly soluble in water.2.3 Preparation of warfarin/ferulic acid rare earth nano-oxide hybrid materials
RE2O3-TDI (20 mg) was dissolved in chloroform (50 mL) and sonicated for 0.5 h. Then, H2N–PEG–NH2 (100 mg) was added and the mixture was stirred at room temperature for 12 h before the addition of warfarin (10 mg) and anhydrous acetone (5 mL). The resulting mixture was refluxed for 12 h under N2 at 70 °C. Upon completion of the reaction, the solid was separated by filtration, washed with diethyl ether (15 mL) and roasted in a vacuum oven at 45 °C for 24 h. The obtained solid was marked as RE2O3-TDI-PEG-W and was soluble in water (>10 mg mL−1) as well as some organic solvents, such as anhydrous ethanol, acetone, DMSO, etc.In a similar manner, ferulic acid (13 mg), dimethylformamide (DMF, 15 mL), N,N′-dicyclohexylcarbodiimide (DCC, 13 mg) and N-hydroxyl succinimide (NHS, 11 mg) were added subsequently to the RE2O3-TDI and H2N–PEG–NH2 mixed solution. The mixture was stirred for 12 h under N2 at room temperature. Finally, the solid was separated by filtration and washed with diethyl ether (15 mL) and heated in a vacuum oven for 24 h at 45 °C. The obtained solid was marked as RE2O3-TDI-PEG-FA and was soluble in water (>10 mg mL−1) and some organic solvents.
2.4 Cell compatibility
The cell compatibility was measured by the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) method.17 The HepG2 cells were routinely cultured in tissue culture boxes with 1640 culture medium (containing 10% fetal bovine serum), and incubated at 37 °C in a humidified atmosphere with 95% air and 5% CO2. After several generations of cells, the solution of hybrid materials (100 μL, 10 mg mL−1, by diluting with 1640 culture medium) was added to the corresponding cell in the well, and then co-cultured at 37 °C for 48 h in a humidified atmosphere with 5% CO2. After harvesting the cells, the MTT solution (150 μL, 5 mg mL−1, PBS buffer solution) was added and the cell was cultured for 4 h at 37 °C. After removing the MTT solution, DMSO (150 μL) was added in each well at 37 °C, and the cell lysate was measured in a Bio-Rad Microplate Reader (model 1680) at excitation and emission wavelengths of 570 nm. The cell activity was expressed as a percentage of the optical absorbance of the test group vs. the control group.172.5 Test of anticlotting activity
3. Results and discussion
3.1 Anticlotting properties
The anticoagulant properties of RE2O3-TDI and RE2O3-TDI-PEG can be attributed to nano RE2O3. RE2O3-TDI-PEG-W/RE2O3-TDI-PEG-FA have longer CT times than that of RE2O3-TDI-PEG, due to the existence of warfarin/ferulic acid. The CT time of Sc2O3-TDI-PEG-W and Sc2O3-TDI-PEG-FA was the longest in all of the hybrid materials because of the similar chemical properties of Sc and Ca. The two elements are neighbours in the same period in the periodic table and the effective nuclear charge of Sc3+ is higher than the other rare earth ions. Therefore, Sc3+ can replace Ca2+ more easily than the other rare earth ions.
The CT and RT of the mixture of drugs and rare earth nano-oxides were also investigated. The results (Table 1) show that the CT and RT of the mixture were shorter than those of the hybrid materials. As there were about 50% (w%) drugs in the mixture, it could not achieve the same anticoagulant performance compared with the hybrid materials (6% warfarin or 4% ferulic acid).
| Mixture | CT/min | P | RT/min | P |
|---|---|---|---|---|
| Sc2O3 + W | 9.20(±0.02) | <0.05 | 6.20(±0.02) | <0.05 |
| Sc2O3 + FA | 9.50(±0.02) | <0.05 | 6.70(±0.02) | <0.05 |
| La2O3 + W | 9.00(±0.02) | <0.05 | 6.10(±0.02) | <0.05 |
| La2O3 + FA | 9.50(±0.02) | <0.05 | 6.50(±0.02) | <0.05 |
| Nd2O3 + W | 10.50(±0.02) | <0.05 | 6.80(±0.02) | <0.05 |
| Nd2O3 + FA | 10.70(±0.02) | <0.05 | 7.30(±0.02) | <0.05 |
| Sm2O3 + W | 9.80(±0.02) | <0.05 | 7.00(±0.02) | <0.05 |
| Sm2O3 + FA | 10.10(±0.02) | <0.05 | 7.50(±0.02) | <0.05 |
| Eu2O3 + W | 9.10(±0.02) | <0.05 | 6.10(±0.02) | <0.05 |
| Eu2O3 + FA | 9.20(±0.02) | <0.05 | 6.20(±0.02) | <0.05 |
| Dy2O3 + W | 9.30(±0.02) | <0.05 | 7.10(±0.02) | <0.05 |
| Dy2O3 + FA | 9.20(±0.02) | <0.05 | 7.30(±0.02) | <0.05 |
| Compound | APTT/s | P | PT/s | P |
|---|---|---|---|---|
| Blank | 27.50(±1) | <0.05 | 12.40(±1) | <0.05 |
| Sc2O3-TDI-PEG-W | 34.20(±2) | <0.05 | 13.60(±1) | <0.05 |
| Sc2O3-TDI-PEG-FA | 33.70(±2) | <0.05 | 13.30(±1) | <0.05 |
| La2O3-TDI-PEG-W | 34.70(±2) | <0.05 | 13.50(±1) | <0.05 |
| La2O3-TDI-PEG-FA | 34.00(±2) | <0.05 | 13.20(±1) | <0.05 |
| Nd2O3-TDI-PEG-W | 36.50(±2) | <0.05 | 13.80(±1) | <0.05 |
| Nd2O3-TDI-PEG-FA | 33.20(±2) | <0.05 | 13.10(±1) | <0.05 |
| Sm2O3-TDI-PEG-W | 33.20(±2) | <0.05 | 13.20(±1) | <0.05 |
| Sm2O3-TDI-PEG-FA | 33.00(±2) | <0.05 | 13.20(±1) | <0.05 |
| Eu2O3-TDI-PEG-W | 34.70(±2) | <0.05 | 13.50(±1) | <0.05 |
| Eu2O3-TDI-PEG-FA | 34.20(±2) | <0.05 | 13.10(±1) | <0.05 |
| Dy2O3-TDI-PEG-W | 34.50(±2) | <0.05 | 13.60(±1) | <0.05 |
| Dy2O3-TDI-PEG-FA | 33.50(±2) | <0.05 | 13.10(±1) | <0.05 |
| Reference | 25-37 | 10-13 |
| Compound | Cell viability (%) | P |
|---|---|---|
| Sc2O3-TDI-PEG-W | 94 | <0.05 |
| Sc2O3-TDI-PEG-FA | 92 | <0.05 |
| La2O3-TDI-PEG-W | 95 | <0.05 |
| La2O3-TDI-PEG-FA | 89 | <0.05 |
| Nd2O3-TDI-PEG-W | 99 | <0.05 |
| Nd2O3-TDI-PEG-FA | 96 | <0.05 |
| Sm2O3-TDI-PEG-W | 93 | <0.05 |
| Sm2O3-TDI-PEG-FA | 88 | <0.05 |
| Eu2O3-TDI-PEG-W | 90 | <0.05 |
| Eu2O3-TDI-PEG-FA | 94 | <0.05 |
| Dy2O3-TDI-PEG-W | 96 | <0.05 |
| Dy2O3-TDI-PEG-FA | 94 | <0.05 |
| Blank | 96 | <0.05 |
3.2 Characterization
Fig. 5(A) shows the IR spectra of Eu2O3-TDI. There was a vibration peak at 3300 cm−1, which indicated the reaction had occurred. However, the presence of a characteristic absorption of –NCO at 2273 cm−1 suggests that one –NCO in the TDI was preserved. This group was used to react with H2N–PEG–NH2.19 The IR spectra of Eu2O3-TDI revealed the presence of the carbonates absorption (1640 and 1543 cm−1),20 the benzene absorption (1605 cm−1), the anti-symmetric and symmetric stretching vibration peaks of the C–H of the TDI methyl (2922 and 2857 cm−1), all of which confirm the successful grafting of TDI onto the surface of nano-Eu2O3.
 | ||
| Fig. 5 IR spectra of (A) Eu2O3-TDI, (B) Eu2O3-TDI-PEG, (C) warfarin (D) Eu2O3-TDI-PEG-W, (E) Ferulic Acid and (F) Eu2O3-TDI-PEG-FA. | ||
Fig. 5(B) shows the IR spectra of Eu2O3-TDI-PEG. The disappearance of the characteristic absorption of –NCO indicates that the –NCO left in the Eu2O3-TDI surface reaction with –NH of PEG. The characteristic vibrations of PEG appeared at 2882 cm−1 (ν(C–C–H)) and 1109 cm−1 (ν(–C–O–C)), which confirmed the formation of Eu2O3-TDI-PEG.
Fig. 5(C) reveals the IR spectra of warfarin. Two carbonyl absorption peaks appeared at 1718 and 1664 cm−1.21 The former belongs to the ketone carbonyl stretching vibration; the latter is the vibration of the aromatic ring of carbonyl stretching, an O–H stretching vibration appeared at 3283–3500 cm−1.
In the IR spectra of Eu2O3-TDI-PEG-W (Fig. 5(D)), the vibration of carbonyl stretching was absent, and –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N absorption appeared at 1631 cm−1, which suggested that the reaction between –C
N absorption appeared at 1631 cm−1, which suggested that the reaction between –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and –NH2 had occurred and that –C
O and –NH2 had occurred and that –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N was formed consequently.
N was formed consequently.
Fig. 5(E) and (F) illustrate the IR spectra of ferulic acid and the hybrid material, Eu2O3-TDI-PEG-FA. The spectra showed that the vibration peak of carboxylic carbonyl appeared at 1686 cm−1 in the hybrid material. This number shifted toward the lower wave number reaching 1635 cm−1, which indicated the formation of an amide bond between ferulic acid and –NH2 of PEG. The results clearly suggest that ferulic acid has been grafted to the surface of nano-Eu2O3. The absorbance of the signal at 3425 cm−1 was the stretching vibration of the phenol hydroxyl group. After forming the hybrid materials Eu2O3-TDI-PEG-FA, it became a smooth wide peak (3300–3600 cm−1), which indicated the successful formation of ferulic acid hybrid materials. The results of the IR spectra of other samples were in approximate agreement with that of Eu2O3-TDI-PEG-W and Eu2O3-TDI-PEG-FA (Table 4).
| Compound | –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N N |
|---|---|---|
| Warfarin | 1718 | — |
| Ferulic acid | 1686 | — |
| Sc2O3-TDI-PEG-W | — | 1627 |
| Sc2O3-TDI-PEG-FA | 1639 | — |
| La2O3-TDI-PEG-W | — | 1641 |
| La2O3-TDI-PEG-FA | 1651 | — |
| Nd2O3-TDI-PEG-W | — | 1647 |
| Nd2O3-TDI-PEG-FA | 1658 | — |
| Sm2O3-TDI-PEG-W | — | 1644 |
| Sm2O3-TDI-PEG-FA | 1656 | — |
| Eu2O3-TDI-PEG-W | — | 1631 |
| Eu2O3-TDI-PEG-FA | 1635 | — |
| Dy2O3-TDI-PEG-W | — | 1638 |
| Dy2O3-TDI-PEG-FA | 1646 | — |
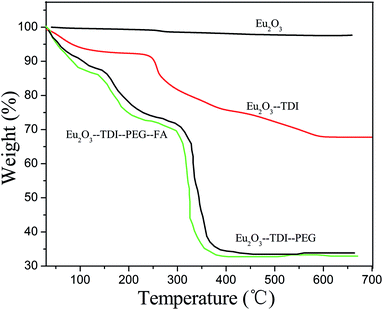
| Compounds | TDI-PEG-W losing temperature/°C, losing rate/% found | TDI-PEG-FA losing temperature/°C, losing rate/% found | Residual weight/% found (calc.) |
|---|---|---|---|
| Eu2O3-TDI-PEG-W | 300–600, 66.7 | 33.3 | |
| Eu2O3-TDI-PEG-FA | 300–600, 65.4 | 34.6 | |
| Sc2O3-TDI-PEG-W | 200–650, 77.5 | 12.5 | |
| Sc2O3-TDI-PEG-FA | 200–650, 78.3 | 13.3 | |
| La2O3-TDI-PEG-W | 4200–550, 70.0 | 29.6 | |
| La2O3-TDI-PEG-FA | 200–550, 74.4 | 25.6 | |
| Nd2O3-TDI-PEG-W | 200–550, 68.8 | 31.2 | |
| Nd2O3-TDI-PEG-FA | 200–550, 67.4 | 32.6 | |
| Sm2O3-TDI-PEG-W | 200–600, 67.6 | 32.4 | |
| Sm2O3-TDI-PEG-FA | 200–600, 72.5 | 26.5 | |
| Dy2O3-TDI-PEG-W | 200–550, 72.2 | 27.8 | |
| Dy2O3-TDI-PEG-FA | 200–550, 71.2 | 26.8 |
Ferulic acid: 3.83 ppm. (–OCH3), 6.37 ppm (–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–benzene), 7.54 ppm (–benzene–CH
C–benzene), 7.54 ppm (–benzene–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 9.57 ppm (–OH), 12.16 ppm. (–COOH); warfarin: 2.12 ppm (–CH3), 4.94 ppm (–CH2), 11.39 ppm. (–OH); H2N–PEG–NH2: 2.49 ppm (–NH2), 3.33 ppm (N–CH2), 3.49 ppm. (–CH2); La-TDI-PEG-FA: 2.32 ppm. (–NH), 6.45 ppm (–CH
C), 9.57 ppm (–OH), 12.16 ppm. (–COOH); warfarin: 2.12 ppm (–CH3), 4.94 ppm (–CH2), 11.39 ppm. (–OH); H2N–PEG–NH2: 2.49 ppm (–NH2), 3.33 ppm (N–CH2), 3.49 ppm. (–CH2); La-TDI-PEG-FA: 2.32 ppm. (–NH), 6.45 ppm (–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–benzene), 7.54 ppm(–benzene–CH
C–benzene), 7.54 ppm(–benzene–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 3.79 ppm (–OCH3), 10.51 ppm (–OH); La-TDI-PEG-W: 2.32 ppm (–CH3), 3.39 ppm (CH–N), 3.49 ppm (–CH2) and 10.45 ppm (–OH).
C), 3.79 ppm (–OCH3), 10.51 ppm (–OH); La-TDI-PEG-W: 2.32 ppm (–CH3), 3.39 ppm (CH–N), 3.49 ppm (–CH2) and 10.45 ppm (–OH).
In La-TDI-PEG-FA, the peak at 12.38 ppm disappeared, which was attributed to the –COOH group in the ferulic acid;22 whereas the signals of –OCH3 of ferulic acid and N–CH2 of H2N–PEG–NH2 protons shifted slightly upfield; in La-TDI-PEG-W, the signals of –CH3 of warfarin at 2.12 ppm and N–CH2 of H2N–PEG–NH2 at 3.33 ppm were moved downfield. Simultaneously, the peak of the –OH group of warfarin at 11.39 ppm shifted upfield to 10.45 ppm. Hence, suggesting the successful preparation of ferulic acid or warfarin was grafted onto nano-RE2O3 modified by H2N–PEG–NH2 and TDI.
The 13C NMR chemical shifts of the main groups of ligands and La-complex list as follows: ferulic acid: 116.74 ppm (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–), 145.37 ppm (–C
C–), 145.37 ppm (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 170.01 ppm (–COOH); warfarin: 23.15 ppm (–C(
C), 170.01 ppm (–COOH); warfarin: 23.15 ppm (–C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)–CH3), 27.12 ppm (–CH3), 47.41 ppm (–CH2); H2N–PEG–NH2: 40.10 ppm (N–CH2), 70.20 ppm (–CH2); La2O3-TDI-PEG-W: 31.11 ppm (–CH3), 36.20 ppm (–C(
O)–CH3), 27.12 ppm (–CH3), 47.41 ppm (–CH2); H2N–PEG–NH2: 40.10 ppm (N–CH2), 70.20 ppm (–CH2); La2O3-TDI-PEG-W: 31.11 ppm (–CH3), 36.20 ppm (–C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)–CH3), 40.17 ppm (N–CH2), 70.21 ppm (–CH2), 162.75 ppm (N–C(
O)–CH3), 40.17 ppm (N–CH2), 70.21 ppm (–CH2), 162.75 ppm (N–C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)–N); La2O3-TDI-PEG-FA: 39.95 ppm (N–CH2), 70.21 ppm (–CH2), 115.09 ppm (C
O)–N); La2O3-TDI-PEG-FA: 39.95 ppm (N–CH2), 70.21 ppm (–CH2), 115.09 ppm (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–), 138.18 ppm (–C
C–), 138.18 ppm (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) and 162.72 ppm (N–C(=O)–N).
C) and 162.72 ppm (N–C(=O)–N).
Obviously, when RE2O3-TDI-PEG-FA was formed, the N–CH2 peak at 40.10 ppm of H2N–PEG–NH2, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– and –C
C– and –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C of ferulic acid, at 116.74 and 145.37 ppm, respectively, shifted weakly upfield. The peak of –COOH was attributed to ferulic acid at 170.01 ppm, which moved to 162.72 ppm because it formed a acylamino group; in La-TDI-PEG-W, the signals of N–CH2 at 40.10 ppm of H2N–PEG–NH2, –C(
C of ferulic acid, at 116.74 and 145.37 ppm, respectively, shifted weakly upfield. The peak of –COOH was attributed to ferulic acid at 170.01 ppm, which moved to 162.72 ppm because it formed a acylamino group; in La-TDI-PEG-W, the signals of N–CH2 at 40.10 ppm of H2N–PEG–NH2, –C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)–CH3 and –CH3 of warfarin at 23.15 and 27.12 ppm, respectively, shifted downfield. All of these phenomena show that RE2O3-TDI-PEG-FA and RE2O3-TDI-PEG-W were successfully synthesized.
O)–CH3 and –CH3 of warfarin at 23.15 and 27.12 ppm, respectively, shifted downfield. All of these phenomena show that RE2O3-TDI-PEG-FA and RE2O3-TDI-PEG-W were successfully synthesized.
| Compounds | PDI | Z-Average (d, nm) | P |
|---|---|---|---|
| Eu2O3-TDI-PEG-W | 0.533 | 181 | <0.5 |
| Eu2O3-TDI-PEG-FA | 0.479 | 157 | <0.5 |
| Sc2O3-TDI-PEG-W | 0.588 | 134 | <0.5 |
| Sc2O3-TDI-PEG-FA | 0.578 | 120 | <0.5 |
| La2O3-TDI-PEG-W | 0.680 | 174 | <0.5 |
| La2O3-TDI-PEG-FA | 0.494 | 156 | <0.5 |
| Nd2O3-TDI-PEG-W | 0.491 | 238 | <0.5 |
| Nd2O3-TDI-PEG-FA | 0.446 | 214 | <0.5 |
| Sm2O3-TDI-PEG-W | 0.468 | 224 | <0.5 |
| Sm2O3-TDI-PEG-FA | 0.442 | 198 | <0.5 |
| Dy2O3-TDI-PEG-W | 0.577 | 268 | <0.5 |
| Dy2O3-TDI-PEG-FA | 0.469 | 247 | <0.5 |
| PEG-4000 | 0.494 | 116 | <0.5 |
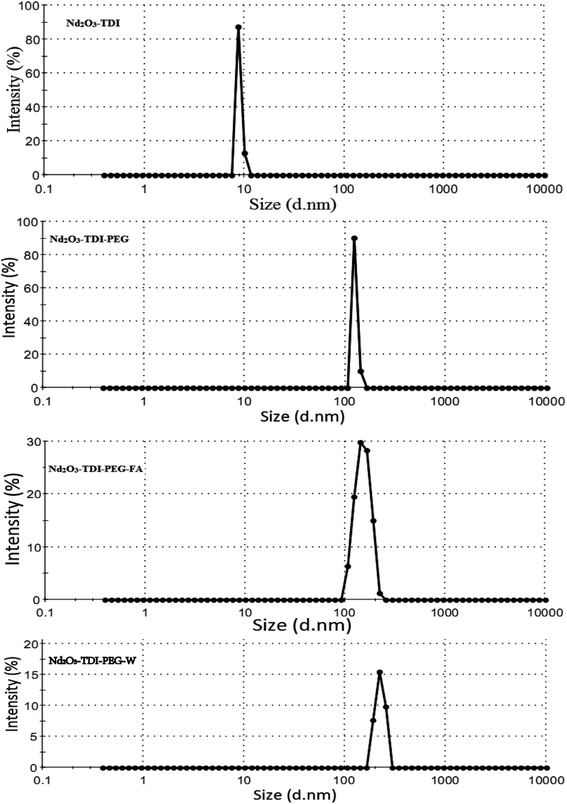
Fig. 10 present the intensity (%) of the particle size for Nd2O3-TDI, Nd2O3-TDI-PEG, Nd2O3-TDI-PEG-W and Nd2O3-TDI-PEG-FA (0.01 mg mL−1, water), respectively. The value of the Z-average is 8.90 nm for Nd2O3-TDI, 200 nm for Nd2O3-TDI-PEG and 238/214 nm for Nd2O3-TDI-PEG-W/Nd2O3-TDI-PEG-FA. From Nd2O3, to hybrid materials, the Z-average value becomes larger and larger, so warfarin (W) or ferulic acid (FA) has been successfully grafted onto the nano-RE2O3 by PEG.
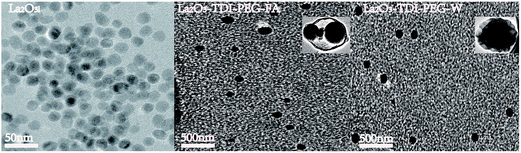
Generally, it is believed when the value of PDI < 0.2, it is mainly a monodisperse system.24 However, the PDI values of all the samples are mostly over 0.2, which suggests that there are different sizes of particle existing in the test samples. This phenomenon is supported by the results of SEM and TEM images.
4. Conclusions
A class of water-soluble anticoagulant warfarin/ferulic acid grafted rare earth nano-oxide hybrid materials was reported by using TDI and PEG as connection reagents. The products were characterized by IR spectroscopy, TGA, 1H and 13C NMR spectroscopy, XRD, SEM, TEM and particle size testing. The results showed that warfarin/ferulic acid were successfully grafted onto the surface of the nano rare earth oxides. It was demonstrated that hybrid materials have better cell compatibility and anticoagulant properties. The anticoagulant time, the recalcification time, the activated partial thromboplastin time and prothrombin time depend on the concentration and therefore the higher the concentration of the hybrid material, the better the anticoagulant property. When a small amount of hybrid material was added to the blood, no obvious variation of the properties of the blood was observed. These novel hybrid materials can serve as a foundation for clinical screening of anticoagulant drugs.References
- E. Nomun, A. Kashiwada and A. Hosoda, Bioorg. Med. Chem., 2003, 11, 3807 CrossRef.
- Y. J. Hu, O. Y. Yu and C. M. Dai, Biomacromolecules, 2010, 11, 106 CrossRef CAS PubMed.
- T. B. Lu, M. Thomas and K. Shelley, J. Med. Chem., 2010, 53, 1843 CrossRef CAS PubMed.
- S. M. Wang, B. Richard and B. Tob, J. Med. Chem., 2010, 53, 1465 CrossRef CAS PubMed.
- S. Y. Ou and K. C. Kwok, J. Sci. Food Agric., 2004, 84, 1261 CrossRef CAS.
- C. Anselmi, M. Centini and M. Ricci, et al., J. Pharm. Biomed. Anal., 2006, 40, 875 CrossRef CAS PubMed.
- B. H. Wang and J. P. Ou-Yang, Cardiovasc. Drug Rev., 2005, 23, 161 CrossRef CAS PubMed.
- T. Q. Jiao, J. G. Wu and F. L. Zeng, Synth. React. Inorg. Met.-Org. Chem., 1999, 29, 725 CrossRef CAS.
- Y. L. Dong, N. N. Luan, H. Y. Wang and Y. M. Song, Acta Chim. Sin., 2008, 66, 1497 CAS in Chinese.
- P. Y. Wang, B. Wu and C. X. Bian, Chin. J. Inorg. Chem., 2012, 28, 1609 CAS in Chinese.
- T. Funakoshi, K. Furushima and H. Shimada, Biochem. Int., 1992, 28, 113 CAS.
- L. J. Fu, J. X. Li, X. G. Yang and K. Wang, J. Biol. Inorg. Chem., 2009, 14, 219 CrossRef CAS PubMed.
- K. J. Wang, H. X. Li and Y. M. Song, Biopolymers, 2010, 93, 887 CrossRef CAS PubMed.
- N. Xiang, X. M. Zhang and L. Y. Tian, J. Biol., 2009, 26, 64 Search PubMed.
- X. B. Zhou and Y. Z. Wei, Chin. Bull. Life Sci., 1999, 11, 70 Search PubMed in Chinese.
- B. D. Wang and C. J. Xu, J. Am. Chem. Soc., 2008, 130, 14437 Search PubMed.
- X. F. Hu and J. Ji, Acta Polym. Sin., 2009, 8, 828 CrossRef.
- S. Kunar, N. Alnasif and E. Fleige, et al., Eur. J. Pharm. Biopharm., 2014, 88, 625 CrossRef PubMed.
- C. M. Wu, T. W. Xu and W. H. Yang, J. Membr. Sci., 2003, 216, 269 CrossRef CAS.
- H. R. Li, J. Lin and H. J. Zhang, Chem. Mater., 2002, 14, 3651 CrossRef CAS.
- E. J. Valente, E. C. Lingafeler and W. R. Porter, J. Med. Chem., 1997, 20, 1489 CrossRef.
- M. Bunzel, J. Ralph, C. Funk and H. Steinhart, Eur. Food Res. Technol., 2003, 217, 128 CrossRef CAS PubMed.
- G. Zingone and F. Rubessa, Int. J. Pharm., 2005, 291, 5 CrossRef PubMed.
- H. B. Bohidar and M. Behboudnia, Colloids Surf., A, 2001, 178, 313 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra14633f |
| This journal is © The Royal Society of Chemistry 2015 |