Ultra-thin C3N4 nanosheets for rapid charge transfer in the core–shell heterojunction of α-sulfur@C3N4 for superior metal-free photocatalysis under visible light†
Xueming Dang,
Xiufang Zhang*,
Weiqiang Zhang,
Xiaoli Dong,
Guowen Wang,
Chun Ma,
Xinxin Zhang,
Hongchao Ma and
Mang Xue
School of Light Industry and Chemical Engineering, Dalian Polytechnic University, Dalian, 116034, China. E-mail: zhangxf@dlpu.edu.cn; Fax: +86 411 86323736; Tel: +86 411 86323508
First published on 7th January 2015
Abstract
The core–shell heterojunctions of ultra-thin C3N4 nanosheet enwrapping spherical α-S composites (α-S@C3N4) were fabricated via a self-assembly process by electrostatic force to realize enhanced photocatalytic ability under visible light. The photocatalytic ability can be adjusted by tuning the amount of the ultra-thin C3N4 nanosheet. The α-S@C3N4 composite with 35% composition of C3N4 nanosheet has the highest photocatalytic ability. The degradation rate of Rhodamine B (RhB) with α-S@C3N4 (35% C3N4) is 6.72 times faster compared to α-S as photocatalyst. This increase could be attributed to the efficient photogenerated holes and electrons separation by the heterojunction, which has excellent charge transfer ability arising from the ultra-thin C3N4 nanosheet. The stability of the α-S is also largely improved by the heterojunction construction.
1. Introduction
Recently, more attention has been paid to semiconductor photocatalysis in the field of pollution elimination as a “green” technology that can completely mineralize most types of pollutants under the illumination of sunlight and moderate ambient conditions.1–3 Development of an efficient visible-light-driven semiconductor material is an important need for practical application of this technology. With unique advantages of low-cost, abundance, and easy access, metal-free elemental photocatalysts are emerging and have great potential for application in industry.4–6 Most recently, Liu et al. demonstrated that α-sulfur (α-S) can be a candidate for visible-light-driven photocatalysis because of its capability of degrading Rhodamine B (RhB) under visible light illumination.7 Since then, to enhance the photocatalytic ability of α-S, some studies have been carried out. Yu and co-workers8 constructed a heterojunction of graphene and g-C3N4 enwrapped α-S, which exhibited enhanced photocatalytic ability for bacterial inactivation. Peng et al.9 reported the fabrication of a sulfur/graphene composite, in which the incorporation of graphene enhanced its photocatalytic activity for the degradation of methyl orange due to the increased hydrophilicity and adsorption capacity. However, similar to the other photocatalysts, α-S still has low quantum efficiency attributed to the limited light absorption ability, the high recombination rate of photogenerated holes and electrons, and the sluggish surface reaction.It is well known that photocatalytic activity is closely related to the size, morphology, structure, and surface area of the photocatalyst.10 Decrease in the size can enhance the photocatalytic activity due to increasing surface area exposed to the light and reactants. As a result, the light absorption and the surface reaction can be improved. Several surfactants have been used as the soft template in the fabrication of photocatalysts for the control of size and morphology.11 One example is poly(vinyl pyrrolidone) (PVP), which has been proved to be an efficient template to either decrease the size or configure the shape of the sphere.12
Fabrication of the heterojunction is another strategy for improving photocatalytic activity potentially attributed to the highly effective charge separation.13 When designing a heterojunction, the critical factor that should be considered is the charge transporting ability of the loading material. One good example is graphene, which is widely used for the construction of the heterojunction due to its extremely high electron mobility.14 Recently, it was reported that bulk g-C3N4 can degrade organic pollutants under visible light irradiation.15–17 It is a layered material and can be exfoliated into an ultra-thin nanosheet with a few layers and even into a single atomic layer nanosheet.18,19 The exfoliated C3N4 nanosheet has a big surface area with very thin layer thickness and excellent conductivity with less resistance between the layers, which make it an excellent material for constructing heterojunctions with other semiconductors. Furthermore, the interface area of the heterojunction is a key parameter that can affect the photocatalytic ability.20 The large contact area can promote charge transfer across the interface, thus increasing the separation of electron–hole pairs and leading to the enhancement of photocatalytic ability. Ideally, the approach to enhance photocatalytic ability is to increase the interface area of the semiconductors at the heterojunction. Therefore, based on this hypothesis, enwrapping the spherical α-S with the selected ultra-thin C3N4 nanosheet to obtain large contact area would improve the catalytic efficiency. Another consideration when fabricating an efficient heterojunction is the matching of the band potential of the semiconductors (denoted as semiconductor 1 and semiconductor 2). When the conduction band (CB) and valence band (VB) potential of semiconductor 1 are lower than those of semiconductor 2, the potential gradient drives photogenerated electrons in the CB of semiconductor 2 to that of semiconductor 1 and photogenerated holes in the VB of semiconductor 1 to that of semiconductor 2. Therefore, the electrons and holes are further separated and stored in different semiconductors, and the recombination of them can be suppressed. It is reported that the VB of α-S is 0.25 eV higher than that of anatase TiO2.7 The band gap of α-S is 2.79 eV. Therefore, the calculated CB and VB of α-S are −0.40 and 2.39 eV vs. NHE, respectively. The CB and VB level of g-C3N4 are −1.3 and 1.4 V vs. NHE, respectively.21 Considering the match of the CB and VB levels with those of α-S, ultra-thin C3N4 is suitable for fabricating a heterojunction with α-S.
In this study, the core–shell heterojunctions of an ultra-thin C3N4 nanosheet enwrapping a spherical α-S (α-S@C3N4) was fabricated, for the first time, for enhanced photocatalytic ability under visible light illumination. The ultra-thin C3N4 nanosheet was used as an efficient charge transporter in the heterojunction structure. Based on the rapid charge transfer, big contact surface area and matching VB and CB levels of the two semiconductors, enhanced photocatalytic ability was expected. RhB, a common pollutant in the industrial wastewater, was selected as a test substance to evaluate the photocatalytic performance of the as-prepared samples.
2. Experimental section
Sample preparation
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) with vigorous stirring, and the resulting PVP solution was noted as solution B. In the following step, solution B was added to solution A followed by stirring for 5 minutes. The resulting mixture was heated to 70 °C followed by the addition of 12 mL of concentrated hydrochloric acid. The temperature was then maintained at 70 °C for 5 min with magnetic stirring. The products were collected by filtration and washed with distilled water until the pH reached 7. The collected samples were then dried at 60 °C for 2 h.
3) with vigorous stirring, and the resulting PVP solution was noted as solution B. In the following step, solution B was added to solution A followed by stirring for 5 minutes. The resulting mixture was heated to 70 °C followed by the addition of 12 mL of concentrated hydrochloric acid. The temperature was then maintained at 70 °C for 5 min with magnetic stirring. The products were collected by filtration and washed with distilled water until the pH reached 7. The collected samples were then dried at 60 °C for 2 h.Sample characterization
The thickness and the morphology of the C3N4 nanosheets were measured by atomic force microscopy (AFM Agilent Pico Plus) and transmission electron microscopy (TEM; JEM-2100(UHR) JEOL). X-ray diffraction (XRD) spectra were recorded by a Rigaku D/MAX-2400 with Cu Kα radiation, with an accelerating voltage of 40 kV and current of 30 mA. The scanning rate was 8° (2θ) min−1, and the scanning range was 10°–80°. Light absorption intensities were measured using a UV-vis spectrophotometer (Shimadzu, UV-2450) with a wavelength range of 200–800 nm. The morphology of the heterojunction composites was obtained with a field emission scanning electron microscope (FE-SEM, Hitachi S-4800) equipped with an energy dispersive spectrometer (EDS) using a voltage of 5 kV, and the structure of the heterojunction composites was characterized with a high applied voltage of 15 kV and by EDS. Fluorescence (FL) spectra were recorded by an FL spectrometer (LS-55, PE). The specific surface area was determined by an adsorption instrument (Tristar 3000) and calculated using the linear portion of the Brunauer–Emmett–Teller (BET) model.Measurement of photocatalytic activity
The photocatalytic activities of the photocatalyst products were monitored through the degradation of RhB under visible light irradiation. The photocatalytic reactions were conducted in a 100 mL cuboid quartz reactor. A 300 W Xe lamp was used as the visible light source. The light was passed through a filter to shield any wavelength below 400 nm. The used light intensity was 30 mW cm−2. In all the experiments, 0.08 g of the photocatalyst was added to 80 mL of a 5 mg L−1 aqueous RhB solution. During each photocatalytic experiment, 5 mL of the suspension was collected at predetermined time intervals. The suspension was centrifuged at 9500 rpm for 10 min, and the concentration of RhB was determined by measuring the absorbance at λ = 554 nm with a Shimadzu UV2000 spectrophotometer. The used photocatalyst was separated from the suspension, washed with deionized water and ethanol three times, and then dried at 60 °C for 6 h for the next experimental run.3. Results and discussion
Analyses of C3N4 nanosheet characterization
The collective analyses indicated that the ultra-thin C3N4 nanosheets were obtained by exfoliation. The TEM image illustrates the highly transparent feature of the C3N4 nanosheets (Fig. 1(a)), indicating the ultra-thin thickness of the nanosheets. The thickness of the nanosheets is 0.8–1.2 nm, as determined by the analysis of the AFM image (Fig. 1(b)). Because the theoretical distance between each layer of the bulk g-C3N4 is 0.326 nm,23,24 it is assumed that two or three layers of the ultra-thin C3N4 nanosheet was successfully fabricated. The XRD patterns were recorded and are shown in Fig. 1(c). In the XRD pattern of bulk g-C3N4, the (002) diffraction at around 27.5° relates to the characteristic interlayer stacking structure, while the (100) diffraction at 13.1° indicates the interplanar structural packing. In the pattern of the ultra-thin C3N4 nanosheet, the (002) diffraction related to the interlayer stacking is also found. However, the intensity is significantly less after exfoliation. This further demonstrated that the thickness of the C3N4 has been largely decreased.Analyses of α-S@C3N4 heterojunction characterization
| αhν = A(hν − Eg)n/2 | (1) |
 | ||
| Fig. 4 (a) UV-vis absorption spectra and (b) calculation of the band gap by Kubelka–Munk function of the α-S, C3N4 nanosheets, S1, S2 and S3. | ||
The calculated band gap is 2.80 eV (Fig. 4(b)), similar to previously reported values. The ultra-thin C3N4 nanosheet can absorb light from 200 to about 410 nm. Its exact band gap is 3.03 eV, which is obviously smaller than that of bulk g-C3N4 (2.70 eV). The similar increase of the band gap has been reported previously and is presumably attributed to the decrease of conjugation length and the strong quantum confinement effect from the ultra-thin structure of the prepared C3N4 nanosheets.18,25 The increased band gap of the ultra-thin C3N4 nanosheet is probably due to the negative shift of its CB and/or the positive shift of its VB. The VB and CB potentials of the ultra-thin C3N4 nanosheets were estimated based on the band gap difference (0.43 eV) of the ultra-thin C3N4 nanosheet and the bulk g-C3N4 from the VB and CB levels of the bulk g-C3N4. The VB potential is between 1.4 and 1.83 eV, and the CB potential is between −1.73 and −1.3 eV. The calculated band gap of S1, S2 and S3 are 2.78, 2.72 and 2.76 eV, respectively (shown in Fig. 4(b)). Compared to α-S (2.8 eV), the decreases of the band gaps are very minimal and can be ignored.
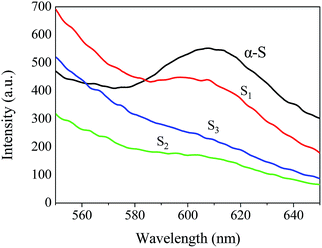
The PL intensities of S1, S2 and S3 are different. The intensity of S2 is similar to that of S3, which is lower than that of S1. From the perspective of the separation ability of photogenerated carriers, there is an optimal thickness of the coating layer in the heterojunction.26 Compared to S1, the C3N4 nanosheet layer of S2 is thicker but still within the optimal thickness. Thus, the separation ability of the S1 for photogenerated holes and electrons of S1 is lower than that of S2. Abundant C3N4 nanosheets loaded on S1 increase the thickness of the C3N4 nanosheet layer to thicker than the optimal thickness. Thus, the separation efficiency of the electrons and holes is not enhanced.
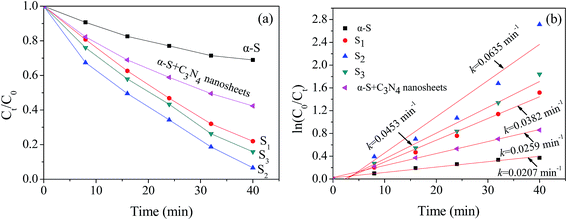
| ln(C0/Ct) = kt | (2) |
Possible mechanisms for the enhanced photocatalytic ability of α-S@C3N4 under visible light illumination are proposed (Fig. 7). Once illuminated by the visible light, the α-S and ultra-thin C3N4 nanosheet are excited. Because the CB (between −1.73 and −1.3 eV vs. NHE) of the ultra-thin C3N4 nanosheet is more negative than that of α-S, the photogenerated electrons in the CB of the C3N4 nanosheet are ejected to the CB of α-S. At the same time, the holes generated by α-S are transported to the VB of the C3N4 nanosheet due to the positive potential of α-S (between 1.4 and 1.83 eV vs. NHE). The matching values of the CB and VB potential between the two semiconductors keeps the electrons and holes separated. Therefore, the recombination of electron–hole pairs is restrained and more electrons and holes can transfer to the surface of the composite and join in the surface reaction. Thus, the photocatalytic activity can be enhanced. The oxidizing species in the photocatalytic degradation of RhB with α-S and α-S@C3N4 are also probed (Fig. S2†). Compared with α-S, the heterojunction construction changes the photocatalytic degradation pathway of RhB. ˙OH radicals no longer serve as oxidizing species with α-S@C3N4 as catalyst, and only holes and ˙O2− radicals contribute to the degradation of RhB. The data and detailed discussion can be read in the ESI.†
Stability is one of the key parameters for evaluating the performance of a photocatalyst. Fig. 8 shows the cycling performance of α-S and S2 for degrading RhB in a photocatalytic process under visible light illumination. After recycling and reusing in each cycle, the removal rate of α-S dramatically decreases from 31.1% for the 1st cycle to 3.0% for the 5th cycle, indicating that α-S is aging in the photocatalytic process. In contrast, the removal rate of S2 does not decrease obviously, inferring that coating C3N4 nanosheets on α-S can largely enhance the stability of α-S. Based on the results listed above, the α-S@C3N4 heterojunction composite is proven to be a good candidate for an efficient, recyclable and stable photocatalyst.
4. Conclusions
The core–shell heterojunctions of α-S@C3N4 were successfully fabricated via a self-assembly process by electrostatic force. The enhancement of the photocatalytic ability of the α-S@C3N4 composite could be attributed to the efficient separation of photogenerated holes and electrons by the heterojunction, which has excellent charge transfer ability arising from the ultra-thin C3N4 nanosheet. The stability of the α-S is largely enhanced by the heterojunction construction. The excellent photocatalytic ability and stability of α-S@C3N4 composite demonstrates its great potential for usage in environmental remediation technology as a highly efficient photocatalyst.Acknowledgements
This work was supported by the National Science Fund China (project no. 21107007) and Cultivation Program for Excellent Talents of Science and Technology Department of Liaoning Province (no. 2014026009).References
- L. F. Yin, J. F. Niu, Z. Y. She and J. Chen, Environ. Sci. Technol., 2010, 44, 5581–5586 CrossRef CAS PubMed.
- X. D. Zhang, W. M. Liao, W. Wu, D. J. Zheng, Y. S. Zhou, B. L. Xue, W. Liu, Z. Q. Lin and Y. L. Deng, J. Mater. Chem. A, 2014, 2, 11035–11039 CAS.
- M. D. Ye, M. Y. Wang, D. J. Zheng, N. Zhang, C. J. Lin and Z. Q. Lin, Nanoscale, 2014, 6, 3576–3584 RSC.
- Z. H. Kang, C. H. A. Tsang, N. B. Wong, Z. D. Zhang and S. T. Lee, J. Am. Chem. Soc., 2007, 129, 12090–12091 CrossRef CAS PubMed.
- Y. D. Chiou and Y. J. Hsu, Appl. Catal., B, 2011, 105, 211–219 CrossRef CAS PubMed.
- F. Wang, W. K. H. Ng, J. C. Yu, H. J. Zhu, C. H. Li, L. Zhang, Z. F. Liu and Q. Li, Appl. Catal., B, 2012, 111, 409–414 CrossRef PubMed.
- G. Liu, P. Niu, L. C. Yin and H. M. Cheng, J. Am. Chem. Soc., 2012, 134, 9070–9073 CrossRef CAS PubMed.
- W. J. Wang, J. C. Yu, D. H. Xia, P. K. Wong and Y. C. Li, Environ. Sci. Technol., 2013, 47, 8724–8732 CAS.
- W. C. Peng and X. Y. Li, Nano Res., 2013, 6, 286–292 CrossRef CAS.
- M. R. Hoffmann, S. T. Martin, W. Choi and D. W. Bahemann, Chem. Rev., 1995, 95, 69–96 CrossRef CAS.
- Y. D. Liu, J. Goebla and Y. D. Yin, Chem. Soc. Rev., 2013, 42, 2610–2653 RSC.
- X. J. Dai, Y. S. Luo, W. D. Zhang and S. Y. Fu, Dalton Trans., 2010, 39, 3426–3432 RSC.
- Q. Xiang, J. Yu and M. Jaroniec, Chem. Soc. Rev., 2012, 41, 782–796 RSC.
- X. F. Zhang, X. Quan, S. Chen and H. T. Yu, Appl. Catal., B, 2001, 105, 237–242 CrossRef PubMed.
- X. C. Wang, K. Maeda, A. Thomas, K. Takanabe, G. Xin, J. M. Carlsson, K. Domen and M. Antonietti, Nat. Mater., 2009, 8, 76–80 CrossRef CAS PubMed.
- K. Maeda, X. C. Wang, Y. Nishihara, D. L. Lu, M. Antoniett and K. Domen, J. Phys. Chem. C, 2009, 113, 4940–4947 CAS.
- S. C. Yan, Z. S. Li and Z. G. Zou, Langmuir, 2009, 25, 10397–10401 CrossRef CAS PubMed.
- J. Xu, L. W. Zhang, R. Shi and Y. F. Zhu, J. Mater. Chem. A, 2013, 1, 14766–14772 CAS.
- H. X. Zhao, H. T. Yu, X. Quan, S. Chen, H. M. Zhao and H. Wang, RSC Adv., 2014, 4, 624–628 RSC.
- S. Chu, X. M. Zheng, F. Kong, G. H. Wu, L. L. Luo, Y. Guo, H. L. Liu, Y. Wang, H. X. Yu and Z. G. Zou, Mater. Chem. Phys., 2011, 129, 1184–1188 CrossRef CAS PubMed.
- S. Chu, Y. Wang, Y. Guo, J. Y. Feng, C. C. Wang, W. J. Luo, X. X. Fan and Z. G. Zou, ACS Catal., 2013, 3, 912–919 CrossRef CAS.
- Y. J. Zhang, A. Thomas, M. Antonietti and X. C. Wang, J. Am. Chem. Soc., 2009, 131, 50–51 CrossRef CAS PubMed.
- K. Schwinghammer, B. Tuffy, M. B. Mesch, E. Wirnhie, C. Martineau, F. Taulelle, W. Schnick, J. Senker and B. V. Lotsch, Angew. Chem., Int. Ed., 2013, 52, 2435–2439 CrossRef CAS PubMed.
- J. Zhang, M. Zhang, R. Q. Sun and X. Wang, Angew. Chem., Int. Ed., 2012, 51, 10145–10149 CrossRef CAS PubMed.
- X. D. Zhang, X. Xie, H. Wang, J. J. Zhang, B. C. Pan and Y. Xie, J. Am. Chem. Soc., 2013, 135, 18–21 CrossRef CAS PubMed.
- H. Wang, X. Quan, H. T. Yu and S. Chen, Carbon, 2008, 46, 1126–1132 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra14623a |
| This journal is © The Royal Society of Chemistry 2015 |