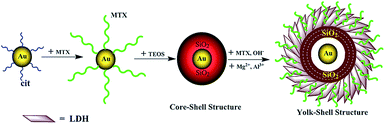
Synthesis of Au yolk/LDH shell nanoparticles as anticancer vehicles†
Xiaolei Huo,
Chaofan Dai,
Shuping Li* and
Xiaodong Li*
Jiangsu Key Laboratory of Biofunctional Material, College of Chemistry and Material Science, Nanjing Normal University, Nanjing, 210023, China. E-mail: lishuping@njnu.edu.cn
First published on 22nd December 2014
Abstract
In this paper, anticancer drug methotrexate (MTX) is applied as a coupling agent to fabricate Au@SiO2 core–shell structures for the first time. Then, a novel kind of Au yolk/LDH shell nanoparticles is obtained by using Au@SiO2 as the template. Finally, the in vitro cytotoxicity is examined and the result indicates that the yolk/shell nanovehicles loaded with MTX exhibit superior anticancer efficacy, compared to the free drug.
During the past decades, remarkable progress has been achieved in developing nanotechnology platforms for early cancer diagnosis and therapy.1 Nanocomposites with both drug carrier and optically active plasmonic components have potential biomedical applications for therapy and imaging diagnosis etc.2 The gold nanoparticles (NPs) with their novel surface plasmon resonance (SPR) have been shown to be available in biolabeling and optical imaging,3 and even for therapeutic applications, such as laser-induced photothermal ablation of cancer cells.4 Layered double hydroxides (LDH) are a kind of inorganic material with positive layer charge which have been widely used as drug and biomaterial delivery systems. For example, many pharmaceutical drugs, such as fenbufen, diclofenac, uorouracil (5FU), methotrexate (MTX) and camptothecin etc. have been intercalated into LDH interlayers to form drug/LDH nanohybrids.5 LDH nanovehicles incorporating drugs or biomolecules and at the same time being functionalized with the luminescent properties of Au nanoparticles can be used for early diagnosis and therapy in clinics.
In this paper, the synthesis of Au yolk/LDH shell nanoparticles, i.e., luminous core locating within hollow LDH shells, is reported. The schematic procedure for the synthesis process is shown in Scheme 1. As known from it, our initial effort is focused on the preparation of gold nanoparticles (Au-NPs) and the seeded growth strategy for the synthesis of citrate-stabilized Au (Au-cit) NPs is applied (Fig. 1A). After that, Au NPs stabilized with another coupling agent of MTX by adding MTX into Au-cit NPs solution and stirring for 24 h. The chemical structure of MTX is shown in Fig. S1.† There is no color change in the whole process, indicating MTX-stabilized Au NPs (also named as Au-MTX) are very stable6 and this could be proved by TEM image of Fig. 1B, i.e., the monodispersity of Au-MTX NPs is even improved compared with that of Au-cit NPs. Shown from Fig. 1B, the diameter of Au-MTX is about 20 nm.
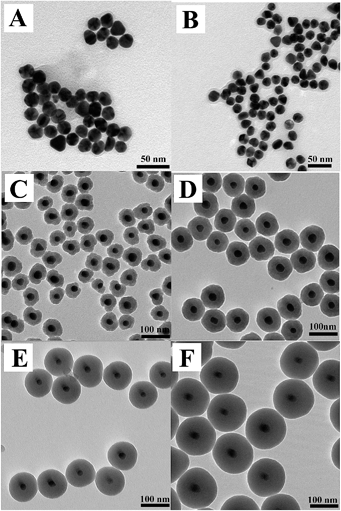 | ||
| Fig. 1 TEM images of Au-cit (A), Au-MTX (B), Au@SiO2 particles prepared with various amounts of TEOS (C) 5 μL, (D) 10 μL, (E) 15 μL, (F) 20 μL. | ||
In order to further fabricate Au@SiO2 core–shell structure, a fixed amount of Au-MTX NPs' solution is centrifuged and redispersed in water again and then added into a mixed solution of ammonia and 2-pronanol. It must be mentioned that there is no loss of particle stability in the whole process, contrary to the condition of Au-cit, i.e., the solution turned easily from red to blue when Au-cit NPs are added into the above mixed solution. In quick succession, various amounts of tetraethyl orthosilicate (TEOS) is added and after 17 h of reaction at room temperature the Au NPs have been coated with amorphous silica shells (Fig. 1C–F) and it can be found that all particles exhibit good monodispersity. The diameter is increasing with the TEOS added. For comparison, the final sample by using Au-cit NPs for SiO2 coating is heavily aggregated and the Au NPs are not coated at all (Fig. S2†). Obviously, MTX actually have a clear superiority for SiO2 coating on Au NPs. However, the Au@SiO2 NPs are not round and uniform when 5 μL TEOS is added.
Afterwards, the Au@SiO2 particles with ∼80 nm in diameter are chosen to study their dissolution process in the solvent of ethanol and water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) in presence of PVP. NaOH is used to adjust the pH value and then the solution is heated to 90 °C to dissolve the coat of SiO2. When the pH value is adjusted to 11.0, the SiO2 coat is absolutely dissolved and only Au NPs leave alone (Fig. 2A). While under the condition of pH = 10.5, the SiO2 coat is partly dissolved and two situations appear, i.e., most of the particles show a special Au yolk/SiO2 shell structure and the shell is very loose; while few coagulated Au NPs without SiO2 shell are also coexisting at the same time (Fig. 2B). Furthermore, the pH values at 10.5 and 11.0 are chosen and micro-controlled to fabricate Au yolk/LDH shell nanoparticles. As described in Scheme 1, the mixed solution containing Mg2+, Al3+ and MTX is dropped into Au@SiO2 system under suitable pH value, to fabricate Au@LDH-MTX structure. The resultant product shows disordered distribution of LDH at high pH (Fig. 2C), while the nanoparticles have an expected structure of Au yolk/LDH shell at suitable value of pH = 10.5 (see Fig. 2D, also named as Au@LDH-MTX). Meanwhile, free Au NPs are not found in the latter situation. This may be attributed to the fact that two kinds of structure, i.e., loose structure of remanent SiO2 together with the formation of LDH sheets, overlapping or crossing, forms a hollow barrier to prevent the escape of Au NPs. The novel yolk/shell structure has an average diameter of ∼120 nm with Au NPs inside. Obviously, the void space is coincident with the size of Au@SiO2. Shown from Fig. 1D, the LDH-MTX sheets surrounding Au core exhibit special curl morphology. For comparison, MTX intercalated LDH (named as LDH-MTX, Fig. S3†) hybrids have been prepared and the relevant information has been illuminated in the supporting part of synthesis of LDH-MTX. As expected, LDH-MTX particles are all disc-like, quite different from random curls of LDH sheets in Au@LDH-MTX.
3) in presence of PVP. NaOH is used to adjust the pH value and then the solution is heated to 90 °C to dissolve the coat of SiO2. When the pH value is adjusted to 11.0, the SiO2 coat is absolutely dissolved and only Au NPs leave alone (Fig. 2A). While under the condition of pH = 10.5, the SiO2 coat is partly dissolved and two situations appear, i.e., most of the particles show a special Au yolk/SiO2 shell structure and the shell is very loose; while few coagulated Au NPs without SiO2 shell are also coexisting at the same time (Fig. 2B). Furthermore, the pH values at 10.5 and 11.0 are chosen and micro-controlled to fabricate Au yolk/LDH shell nanoparticles. As described in Scheme 1, the mixed solution containing Mg2+, Al3+ and MTX is dropped into Au@SiO2 system under suitable pH value, to fabricate Au@LDH-MTX structure. The resultant product shows disordered distribution of LDH at high pH (Fig. 2C), while the nanoparticles have an expected structure of Au yolk/LDH shell at suitable value of pH = 10.5 (see Fig. 2D, also named as Au@LDH-MTX). Meanwhile, free Au NPs are not found in the latter situation. This may be attributed to the fact that two kinds of structure, i.e., loose structure of remanent SiO2 together with the formation of LDH sheets, overlapping or crossing, forms a hollow barrier to prevent the escape of Au NPs. The novel yolk/shell structure has an average diameter of ∼120 nm with Au NPs inside. Obviously, the void space is coincident with the size of Au@SiO2. Shown from Fig. 1D, the LDH-MTX sheets surrounding Au core exhibit special curl morphology. For comparison, MTX intercalated LDH (named as LDH-MTX, Fig. S3†) hybrids have been prepared and the relevant information has been illuminated in the supporting part of synthesis of LDH-MTX. As expected, LDH-MTX particles are all disc-like, quite different from random curls of LDH sheets in Au@LDH-MTX.
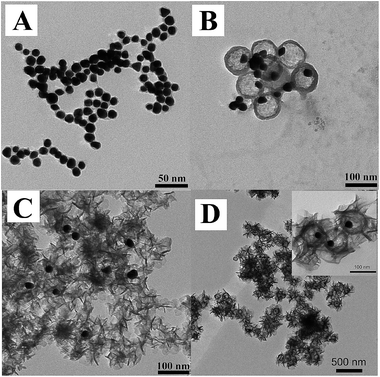 | ||
| Fig. 2 TEM image of Au@SiO2 dissolve at different pH: (A) pH = 11. (B) pH = 10.5 and the synthesis of Au@LDH-MTX at (C) pH = 11. (D) pH = 10.5. | ||
From the UV-vis absorption spectra (Fig. 3A), the free MTX shows three characteristic absorption peaks at 258, 302, and 372 nm. For the Au-MTX and Au@LDH-MTX, the new absorption peaks appear at 530 nm and 532 nm, due to the Au adsorption effect. As for Au@SiO2, only Au adsorption peak at 541 nm is observed. It should be mentioned that three MTX absorption peaks still retain in Au@LDH-MTX, indicating the successful drug loading. FTIR spectra of MTX, LDH-MTX, Au@SiO2 and Au@LDH-MTX are shown in Fig. 3B. As for MTX, the most characteristic absorption band for carboxylate group is due to the antisymmetric COO− vibration in the region of 1570–1660 cm−1.7 An intense peak at 1645 cm−1 for LDH-MTX and Au@LDH-MTX belongs to –NH2 group of MTX, and the bond centered at 1450 cm−1 for the two samples corresponds to the symmetric stretching mode of the COO− group, indicating the successful intercalation of MTX into LDH layers for both LDH-MTX and Au@LDH-MTX. The bending vibrations at 1378 cm−1 in LDH-MTX correspond to the deformation mode of the NO3−, proving the coexistence of NO3− and MTX anions in the interlayer of LDH. Furthermore, the peak centered at 1100 cm−1 for Au@SiO2 and Au@LDH-MTX corresponds to the deformation mode of Si–O, indicating the presence of SiO2 in Au@LDH-MTX.
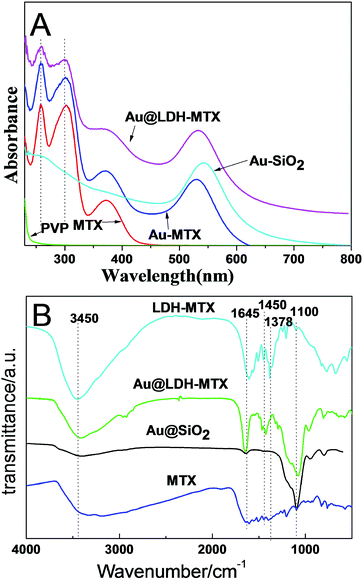 | ||
| Fig. 3 (A) UV-vis absorption spectra of PVP, MTX, Au-MTX, Au@SiO2 and Au@LDH-MTX. (B) FTIR spectra of MTX, LDH-MTX, Au@SiO2 and Au@LDH-MTX. | ||
In our experiment, the functional agent of MTX is served as both the target anticancer drug on LDH sheets and effective surface-coupling agents for SiO2 coating. During the coating progress of SiO2 on Au core, the citrate-stabilized Au NPs are easy to aggregate in the solution containing ammonia and 2-pronanol while the MTX-stabilized AuNPs are stable enough to maintain good monodispersity. Mulvaney et al.8 reported a three-step procedure involving the use of an amineterminated silane as the coupling agent to render the gold surfaces vitreophilic. Graf6 used poly (vinylpyrrolidone) (PVP) as a coupling agent for SiO2 coating on Au NPs. Lu9 coated the citrate-stabilized gold nanoparticles with SiO2 directly under the delicate control of the synthesis condition. Wong10 used 11-mercaptoundecanoic acid (MUA) as ligand to render Au surface amenable for silica adsorption and coating. From their studies, such functional groups as –NH2, –SH or –COO− were used to conjugate to the surface of Au particles, making Au surface easy for further functionalization. From the structure point of view, MTX is an excellent Au stabilizer since MTX contains both diamino and dicarboxyl function groups. Obviously, Au NPs surrounded by sufficient –NH2 and –COO− groups of MTX are favorable for SiO2 coating and the good stability and monodispersity of Au@SiO2 is the best proof. Besides, another advantage of using MTX as the coupling agent is that MTX is the anticancer drug that we want to load in the later steps, thus avoiding the introduction of other impurities.
The anticancer efficacy of Au@LDH-MTX on cancer cells A549 is also investigated by assessing the cytotoxicity using the MTT (a yellow tetrazole) assay.11 The obtained Au@LDH-MTX with around 16% MTX loading (detected by UV-vis adsorption spectra) is dispersed in 5 mL Dubecco's modified eagle medium (DMEM) and the final concentration of MTX is 50 μg mL−1. Fig. 4 shows the cytotoxic effect on the breast cancer cell line (A549) after 24 h and 48 h of treatment with Au@LDH-MTX. Furthermore, the efficacy of the free drug MTX (50 μg mL−1), and LDH-MTX (the concentration of MTX is 50 μg mL−1) is also tested only for comparisons. The results demonstrate that the best cancer cell inhibition could be achieved in the presence of Au@LDH-MTX nanovehicles, which is much better than that of free MTX and LDH-MTX. This result indicates that MTX delivery to the tumor cell is noticeably enhanced by hybridization with LDH sheets and Au core and the excellent anticancer efficacy may be probably attributed to the synergistic effect of the special yolk/shell structure, which still needs deep study.
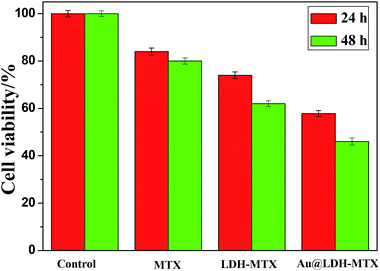 | ||
| Fig. 4 In vitro cell cytotoxicity toward A549 cells after 24 h and 48 h of incubation with free MTX, LDH-MTX and Au@LDH-MTX. | ||
Conclusions
In summary, a facile method to coat silica shells onto Au nanoparticles to form Au@SiO2 core–shell structure is presented. Furthermore, the Au@SiO2 as template is used to get a novel kind of Au yolk/LDH shell nanoparticles. The resultant nanovehicles loaded with MTX exhibited superior anticancer efficacy comparing to the free drug in MTT assay.Acknowledgements
The authors are grateful for the financial support of Project by National Natural Science Foundation of China (21073093 and 21273116), Fund of Priority Academic Program Development of Jiangsu Higher Education Institutions and Jiangsu Collaborative Innovation Center of Biomedical Functional Materials.Notes and references
- L. J. Wang, H. Y. Xing, S. J. Zhang, Q. G. Ren, L. M. Pan, K. Zhang, W. B. Bu, X. P. Zheng, L. P. Zhou, W. J. Peng, Y. Q. Hua and J. L. Shi, Biomaterials, 2013, 34, 3390 CrossRef CAS PubMed.
- D. Qiu, L. Gu, X. L. Sun, D. H. Ren, Z. G. Gu and Z. J. Xu, RSC Adv., 2014, 4, 61313 RSC.
- I. H. EI-Sayed, X. H. Huang and M. A. EI-Sayed, Nano Lett., 2005, 5, 829 CrossRef PubMed.
- P. R. Chandrana and N. Sandhyarani, RSC Adv., 2014, 4, 44922 RSC.
- X. Q. Zhang, M. G. Zeng, S. P. Li and X. D. Li, Colloids Surf., B, 2014, 117, 98 CrossRef CAS PubMed.
- C. Graf, D. L. Vossen, A. Imhof and A. van Blaaderen, Langmuir, 2003, 19, 6693 CrossRef CAS.
- D. Y. Tian, Z. L. Liua, S. P. Li and X. D. Li, Mater. Sci. Eng., C, 2014, 45, 297 CrossRef CAS PubMed.
- L. M. Liz-Marzan, M. Giersig and P. Mulvaney, Langmuir, 1996, 4329 CrossRef CAS.
- Y. Lu, Y. Yin, Z. Y. Li and Y. N. Xia, Nano Lett., 2002, 785 CrossRef CAS.
- Y. J. Wong, L. Zhu, W. S. Teo, Y. W. Tan, Y. H. Yang, C. Wang and H. Y. Chen, J. Am. Chem. Soc., 2011, 11422 CrossRef CAS PubMed.
- P. R. Twentyman and M. Luscombe, Br. J. Cancer, 1987, 56, 279 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra14585b |
| This journal is © The Royal Society of Chemistry 2015 |