Hydrolysis of cellobiose to monosaccharide catalyzed by functional Lanthanum(III) metallomicelle†
Xiao Peng,
Xiang-Guang Meng*,
Chun Mi and
Xiao-Hong Liao
Key Laboratory of Green Chemistry and Technology, Ministry of Education, College of Chemistry, Sichuan University, Chengdu, Sichuan 610064, China. E-mail: mengxgchem@163.com; Fax: +86-28-85412291; Tel: +86-28-85462979
First published on 17th December 2014
Abstract
A novel surfactant, 3-(dodecylimino)butan-2-one-oxime (DMBO), was synthesized. The metallomicelle La(DMBO)2 was prepared and used as a mimic of β-glucosidase to catalyze the hydrolysis of cellobiose in weakly alkaline aqueous solution at relative low temperature (80–110 °C). This study indicated that the functional metallomicelle displayed effective catalytic activity for hydrolysis of cellobiose to monosaccharide (glucose, fructose and 1,6-anhydroglucose) and glucosyl-erythrose. The conversion of cellobiose and selectivity of monosaccharide could reach 38.5% and 71.1%, respectively, for a reaction time of 10 h at pH 9.0 and 95 °C. The possible reaction pathways of cellobiose hydrolysis are proposed and the catalysis reaction rate constant kcat and Michaelis constant Km for the cellobiose hydrolysis were calculated. The apparent activation energy (Ea = 84.6 kJ mol−1) of cellobiose to monosaccharide was evaluated.
1. Introduction
Cellulose, a linear macromolecule connected by β-1,4-glycosidic bonds of D-glucose units, is the most abundant organic compound in nature.1,2 Glucose is an important platform compound to produce biofuels and chemicals such as 5-(hydroxymethyl)furfural, lactic acid and 3-hydroxyacrylic acid.3–6 Therefore hydrolysis of cellulose into glucose is one of the bottlenecks and challenging problems in the field of utilization of biomass.7,8 Although some researchers have reported the hydrolysis of cellulose into glucose through acid catalysts,9–12 subcritical and supercritical water13–16 and ionic liquid,17–19 the problems of low activity and/or selectivity, severe reaction conditions and potential environmental pollution have not been resolved. Cellulase-catalyzed hydrolysis is an ideal process for cellulose degradation owing to the high selectivity of glucose product and the benign conditions.20,21 However, the high cost and instability of the enzyme limit its further application.22 There are three kinds of cellulase for cellulose hydrolysis by synergistic action: β-1,4-endoglucanase (EC3.2.1.4), β-1,4-exoglucanase (EC3.2.1.91) and β-glucosidase (EC3.2.1.21).23,24 β-Glucosidase constitutes a major group of glycoside hydrolase and hydrolyzes cellobiose to glucose efficiently.25,26 The catalytic mechanism of β-glucosidase was believed to consist of two reaction steps involving glycosylation and deglycosylation steps.27,28Functional micelles, a new kind of self-assembly supramolecular system, which could not only simulate the active center but also the hydrophobic microenvironment of enzymes, have attracted much attention of biologists and chemists.29–33 Micelles, especially metallomicelles consisting of a metal and long-chain surfactant micelle, are widely applied to catalyze reactions34–36 and to prepare new materials.37–39 In this paper, a novel metallomicelle, La(DMBO)2, as a mimic of β-glucosidase, was employed to catalyze the hydrolysis of cellobiose, which is the basic structural unit and a good model of cellulose, in weakly alkaline aqueous solution at relative low temperature (80–110 °C). This metallomicelle displayed excellent catalytic activity and selectivity of monosaccharide for cellobiose hydrolysis.
2. Experimental
2.1 Materials
β-D-(+)-Cellobiose was of biological grade and purchased from J&K Corp. Company. Diacetyl monoxime (Damx), dodecylamine, La(NO3)3·6H2O and methanol were of analytical grade and purchased from Kelong Chemical Company and used after certain purification. Acetonitrile was of chromatographic pure grade and purchased from Adamas Company. Whatman 42 filter paper was purchased from GE Healthcare companies.2.2 Synthesis of 3-(dodecylimino)butan-2-one-oxime (DMBO)
Damx (2.525 g) was dissolved in 20 ml absolute methanol, and then the solution was added dropwise into 30 ml methanol solution of dodecylamine (3.70 g), then 0.4 g NaOH was added. The mixture was heated to 70 °C and refluxed for 20 h, then cooled to room temperature. The mixture solution was treated by silica gel column chromatography (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v, ethyl acetate–chloroform) and a yellowish crystalline product was obtained. The yield of DMBO was 3.49 g (65%). The contents of C, H, N and O elements of DMBO were determined using an elemental analyzer (MOD 1106, Carlo Erba Company of Italy). Found: C, 71.62; H, 11.98; N, 10.39; O, 5.93%. Calc. for C16H32N2O: C, 71.64; H, 11.94; N, 10.45; O, 5.97%. 1H NMR (AM-400, Bruker of Switzerland): δ (400 MHz, CDCl3): 11.8 (1H, s, –OH), 1.21–1.52 (22H, m, 11-CH2); 3.15 (3H, s, –CH3CNOH), 2.04 (3H, s, –CH3CN–C12H25); 0.84 (3H, m, –CH3C11H22).
3 v/v, ethyl acetate–chloroform) and a yellowish crystalline product was obtained. The yield of DMBO was 3.49 g (65%). The contents of C, H, N and O elements of DMBO were determined using an elemental analyzer (MOD 1106, Carlo Erba Company of Italy). Found: C, 71.62; H, 11.98; N, 10.39; O, 5.93%. Calc. for C16H32N2O: C, 71.64; H, 11.94; N, 10.45; O, 5.97%. 1H NMR (AM-400, Bruker of Switzerland): δ (400 MHz, CDCl3): 11.8 (1H, s, –OH), 1.21–1.52 (22H, m, 11-CH2); 3.15 (3H, s, –CH3CNOH), 2.04 (3H, s, –CH3CN–C12H25); 0.84 (3H, m, –CH3C11H22).
2.3 Method
The initial reaction solution, containing 0.02 mol·L−1 cellobiose and 0.002 mol·L−1 catalyst, was heated and kept at desired temperature. Before heating, N2 gas was passed into the solution for 30 min for the cases of reaction at temperature below 100 °C. For the cases of reaction above 100 °C, the N2 was passed into reaction solution until the reaction was completed. Samples were extracted from the reactor periodically, and cellobiose and products in the reaction solution were analyzed and their concentrations were determined by HPLC equipped with a RI detector (Shodex 201R, Japan) and a Sugar-D chromatographic column. The pH of solution was adjusted by H2SO4 or NaOH. For the hydrolysis of cellulose (Whatman 42 filter paper), N2 gas was passed into the flask continuously until the reaction was completed, and the total reducing sugar was detected by the DNS method40,41 with a UV-5300 spectrophotometer (Yuanxi Company, China). On a carbon basis, the conversion of cellobiose X was calculated as X = (C0 − Ct)/C0, yield of monosaccharide Y was calculated as Y = C1t/2C0 and the selectivity of monosaccharide S was calculated as S = Y/X, where C0, Ct are the concentrations of cellobiose at reaction time t = 0 and t, respectively, and C1t is the total concentration of monosaccharide (glucose, fructose and 1,6-anhydroglucose) at time t.3. Results and discussion
3.1 Critical micelle concentration of surfactant and Job plots
Surfactant molecules can aggregate to form micelles above the critical micelle concentration (cmc). Both the characteristics and the self-assembly supramolecular structure of a micelle are far different from its monomer.42 In this work the cmc of surfactant DMBO was measured with the electrical conductivity method using a conductivity meter (DDS-307, China). According to plots of electrical conductivity vs. concentrations of DMBO (Fig. S1 in the ESI†), the cmc of DMBO, which corresponds to the intersection of two straight lines, was evaluated as about 1.63 × 10−4 mol L−1.In order to determine the chelating stoichiometry of the reactive metal complex, the kinetic versions of Job plots were utilized, as shown in Fig. S2 in the ESI,† in which the conversion of cellobiose or yield of monosaccharide was plotted as a function of the mole fraction (r) of ligand or La3+ keeping their total concentration constant.30,43 From Fig. S2 in the ESI† it can be seen that the r-values corresponding to the maximum conversion of cellobiose and yield of monosaccharide were all about 0.67, which suggests that the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complex (metal
2 complex (metal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ligand) should be the active species at pH 9.0.
ligand) should be the active species at pH 9.0.
3.2 Comparison of catalytic activity
Cellobiose is stable and difficult to hydrolyze in a near-neutral aqueous solution. However it can be rapidly degraded under strong acid (pH < 2) or alkali (pH > 10) conditions (Fig. S3 in the ESI†). The results of cellobiose hydrolysis catalyzed by different catalytic systems at pH 9.0, 90 °C and 10 h are listed in Table 1, from which we can see that in the absence of any catalyst only 3.7% of cellobiose could degrade under those conditions.| System | Conversion of cellobiose/% | Yield of monosaccharide/% | Selectivity of monosaccharide/% |
|---|---|---|---|
| a [Cellobiose]0 = 0.02 mol L−1, [catalyst] = 0.002 mol L−1, pH 9.0, 90 °C, 10 h. | |||
| Bulk solution | 3.7 | 1.9 | 48.6 |
| Damx | 8.9 | 4.6 | 51.7 |
| DMBO | 11.1 | 5.8 | 52.2 |
| La(Damx)2 | 17.7 | 9.7 | 54.8 |
| La(DMBO)2 | 29.5 | 17.8 | 60.3 |
Damx containing the N–OH functional group in relatively low concentration displayed certain catalytic activity for cellobiose hydrolysis. Metal complex La(Damx)2 clearly enhanced the reaction rate. Interestingly, when a long hydrocarbon chain was grafted onto Damx, micelles formed and showed excellent catalytic activity – probably owing to the enzyme-like microenvironmental effects.30,31 Further, it was also found that the metallomicelle La(DMBO)2 showed the best catalytic activity under the same conditions.
To understand the effect of the structure of the metal complex on reaction activity, several metal complexes (Fig. S4 in the ESI†) were employed as catalysts to catalyze cellobiose hydrolysis under the same conditions. These catalysts showed far lower catalytic activity than La(Damx)2 (Table S1 in the ESI†). This indicated that the strong nucleophilic functional group N–O− may play an important role in the catalysis process under weakly alkaline conditions.
3.3 Effect of pH
From Fig. 1 it can be seen that for the metallomicelle La(DMBO)2-catalyzed system, pH has great influence on conversion of cellobiose and yield of monosaccharide. The conversion of cellobiose increased with increasing pH and reached about 100% for reaction of 10 h at pH 12 and 90 °C. However the variation of the yield of monosaccharide was complex. Initially the yield of monosaccharide increased with increasing pH and reached a maximum at pH 11.5. From Table 2 it can be seen that the main component is glucose. According to our experimental data, glucose and fructose can be rapidly degraded to smaller-molecule substances, including 5-(hydroxymethyl)furfural, laevulinic acid and methanoic acid, under strong alkali conditions (pH > 11.5). | ||
Fig. 1 Plots of conversion of cellobiose ( ) and yield of monosaccharide ( ) and yield of monosaccharide ( ) versus pH at 90 °C for 10 h. ) versus pH at 90 °C for 10 h. | ||
| T/°C | Conversion of cellobiose/% | Yield of monosaccharide | Yield of monosaccharide/% | Yield of GE/% | Selectivity of monosaccharide/% | ||
|---|---|---|---|---|---|---|---|
| Glucose/% | Fructose/% | 1,6-Anhydroglucose/% | |||||
| 80 | 13.5 | 5.2 | 1.1 | 1.2 | 7.5 | 5.7 | 55.6 |
| 85 | 20.8 | 7.7 | 1.6 | 2.6 | 11.9 | 7.8 | 57.2 |
| 90 | 29.5 | 11.4 | 2.5 | 3.9 | 17.8 | 10.5 | 60.3 |
| 95 | 38.5 | 18.5 | 3.4 | 5.5 | 27.4 | 10.1 | 71.1 |
| 100 | 48.6 | 21.5 | 3.9 | 6.1 | 31.5 | 15.3 | 64.8 |
| 110 | 70.4 | 26.0 | 4.7 | 7.7 | 38.4 | 30.3 | 54.5 |
3.4 Effect of temperature
Cellulose is stable and difficult to hydrolyze under mild conditions owing to the large activation energy of its hydrolysis reaction, so the hydrolysis reaction is usually carried out at high temperatures. However glucose can be easily decomposed to smaller-molecule substances, and therefore the selectivity of monosaccharide is generally very low at high temperatures.15,44 In this work, cellobiose hydrolysis reaction was carried out at relatively low temperature (80–110 °C). Fig. 2 shows the effect of temperature on both conversion of cellobiose and yield of monosaccharide. Apparently, higher temperature accelerated the reaction rate of cellobiose hydrolysis. However, it can be further seen that the selectivity of monosaccharide increased gradually with increasing temperature from 80 to 95 °C, and then decreased from 95 to 110 °C. | ||
Fig. 2 Conversion of cellobiose (A) and yield of monosaccharide (B) catalyzed by La(DMBO)2 with time at different temperatures at pH 9.0: ( ) 80 °C, ( ) 80 °C, ( ) 85 °C, ( ) 85 °C, ( ) 90 °C, ( ) 90 °C, ( ) 95 °C, ( ) 95 °C, ( ) 100 °C, ( ) 100 °C, ( ) 110 °C. ) 110 °C. | ||
3.5 Products analysis and possible reaction pathway
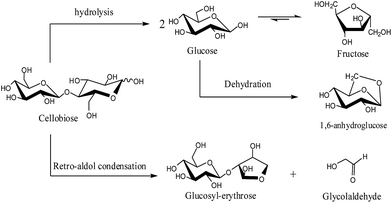
In order to analyze the reaction products, HPLC and pulsed amperometric detection and mass spectrometry (PAD-MS) (LCMS-IT-TOF, Shimadzu of Japan) were employed. These studies indicated that there were four products – namely glucose, fructose, 1,6-anhydroglucose and glucosyl-erythrose (GE) – in the reaction solution, which is illustrated in Fig. 3. For the monosaccharides (glucose, fructose and 1,6-anhydroglucose), their content in the reaction solution was determined by HPLC with the external standard method. An unknown substance, whose mass spectrometry peaks at 283.1 ([GE + H]+) and 304.3 ([GE + Na]+) by PAD-MS(E+) as shown in Fig. S5 in the ESI,† was believed to be GE, which has been widely reported as one of main products of cellobiose decomposition,13,15 although it cannot be accurately identified. The conversion of cellobiose and distribution of products at various temperatures for reaction of 10 h at pH 9.0 are listed in Table 2. Based on these studies, possible reaction pathways of cellobiose hydrolysis were suggested, as illustrated in Scheme 1.3.6 Reaction kinetics
In many previous reports, kinetic behaviors of cellulose and cellobiose degradation were generally described as pseudo-first-order reactions.1,10,13 However, when the association of catalyst (or enzyme) with substrate before further reacting is not ignored, the catalysis reaction involves a more complex kinetic process than simple first-order kinetics. In this work we found the hydrolysis of cellobiose showed complex kinetic characteristics. It seemed that two apparent first-order kinetic processes occurred, as illustrated in Fig. S6 in the ESI;† there were two straight lines in plots of −ln(1 − ct/c0) versus t. It is possible that the reaction product would inhibit the reaction, which resulted in a much slower reaction rate in the later stage than in the early stage of the hydrolysis reaction. Thus, according to our experimental results, a more detailed reaction pathway for cellobiose hydrolysis is proposed as shown in Scheme 2: first, cellobiose S combined with surfactant E to form an intermediate compound ES, and then ES further reacted to monosaccharide M and glucosyl-erythrose GE. In the first step, the surfactant E combined with substrate S reversibly with rate constants k1 and k−1. Then followed the rate-determining steps with rate constants k2 and k3. k4 and k5 are the rate constants of cellobiose hydrolysis to M and GE in bulk solution, respectively. Based on reaction kinetic theory, kinetic eqn (1) and (2) were deduced, and the detailed deduced process can be found in the ESI.†
 | (1) |
 | (2) |
 is the Michaelis constant, kcat = k2 + k3, k0 = k4 + k5, and ET is the total concentration of surfactant. To avoid the interference of reaction products with reaction rate, the data of initial reaction time of 10 h were used to calculate kcat, Km and k2 in this work. Variables Km and kcat were obtained by nonlinear fitting according to eqn S(9) in the ESI.† Variable k2 was calculated according to eqn S(16) in the ESI.† The calculated results are listed in Table 3. From Fig. S7 and S8 in the ESI† it can be seen that the experimental data were in good agreement with the theoretical curves, and both the nonlinear correlation coefficients of eqn S(9) and the linear correlation coefficients of eqn S(16) were all above 0.97. This indicated that the reaction pathways (Scheme 2, eqn (1) and (2)) were reasonable.
is the Michaelis constant, kcat = k2 + k3, k0 = k4 + k5, and ET is the total concentration of surfactant. To avoid the interference of reaction products with reaction rate, the data of initial reaction time of 10 h were used to calculate kcat, Km and k2 in this work. Variables Km and kcat were obtained by nonlinear fitting according to eqn S(9) in the ESI.† Variable k2 was calculated according to eqn S(16) in the ESI.† The calculated results are listed in Table 3. From Fig. S7 and S8 in the ESI† it can be seen that the experimental data were in good agreement with the theoretical curves, and both the nonlinear correlation coefficients of eqn S(9) and the linear correlation coefficients of eqn S(16) were all above 0.97. This indicated that the reaction pathways (Scheme 2, eqn (1) and (2)) were reasonable.
| T/°C | 80 | 85 | 90 | 95 | 100 | 110 |
|---|---|---|---|---|---|---|
| 102 Km/mol−1 L−1 | 2.48 | 2.55 | 2.89 | 3.03 | 3.47 | 3.53 |
| 104 kcat/s−1 | 0.879 | 1.29 | 1.94 | 2.90 | 4.53 | 11.5 |
| 104 k2/s−1 | 0.57 | 0.77 | 1.21 | 2.04 | 2.89 | 4.91 |
From Table 3 it can be seen that values of Km were all relatively small, which indicated the association of catalyst with cellobiose was clearly present in the kinetic reaction process. Although the reaction rate constants kcat and k2 were 100–300-fold smaller than those of catalysis by natural β-glucosidases,20,45 the metallomicelle La(DMBO)2 displayed considerable catalytic activity under these conditions. The hydrolysis mechanism for β-glucosidase was believed to be a two-step displacement process involving glycosylation and deglycosylation steps. In the catalytic active domain, two carboxylic acid groups –COOH and COO− of glutamate residues play an essential role for the catalysis reaction.27,28 In previous reports, organic acid catalysts such as oxalic acid, fumaric acid and maleic acid showed good catalytic activity only for the pretreatment of cellulose.46–48 In our recent studies, we found that micelles containing the carboxylic acid group –COOH showed certain catalytic activity for the hydrolysis of methyl-β-D-cellobioside; however, the micelles displayed catalytic activity only for the glycosylation step rather than for the deglycosylation step.49 In the present work it is found that metallomicelles containing oximido group N–OH displayed excellent catalytic activity for both hydrolysis of cellobiose and selectivity of monosaccharide, although the oximido functional group does not appear in the catalytic active domain of natural cellulase.
The activation energy and pre-exponential factor of the reactions were determined from the Arrhenius plots of ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kcat vs. 1/T and ln
kcat vs. 1/T and ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k2 vs. 1/T, as shown in Fig. 4. The activation energy Ea1 and pre-exponential factor A1 of cellobiose conversion were evaluated as 96.13 kJ mol−1 and 1.36 × 1010 s−1 respectively. The activation energy Ea2 and the pre-exponential factor A2 for generation of monosaccharide were evaluated as 84.6 kJ mol−1 and 1.88 × 108 s−1 respectively, which were obtained easily through the slope and intercept of the linear plots respectively and the correlation coefficients of linear plots were above 0.98. It has been reported that the activation energy Ea of cellobiose hydrolysis catalyzed by enzyme was about 3–50 kJ mol−1,20,50,51 the activation energy of cellobiose hydrolysis catalyzed by non-enzyme was in the range of 110–200 kJ mol−1,1,13,52–55 and the activation energy for generation of monosaccharide was in the range 100–130 kJ mol−1.52,53 The activation energies for the hydrolysis of cellobiose and the generation of monosaccharide in this work are all relatively small, which indicates that the metallomicelle system displayed good catalytic efficiency for the breakage of β-glycoside bonds under mild conditions.
k2 vs. 1/T, as shown in Fig. 4. The activation energy Ea1 and pre-exponential factor A1 of cellobiose conversion were evaluated as 96.13 kJ mol−1 and 1.36 × 1010 s−1 respectively. The activation energy Ea2 and the pre-exponential factor A2 for generation of monosaccharide were evaluated as 84.6 kJ mol−1 and 1.88 × 108 s−1 respectively, which were obtained easily through the slope and intercept of the linear plots respectively and the correlation coefficients of linear plots were above 0.98. It has been reported that the activation energy Ea of cellobiose hydrolysis catalyzed by enzyme was about 3–50 kJ mol−1,20,50,51 the activation energy of cellobiose hydrolysis catalyzed by non-enzyme was in the range of 110–200 kJ mol−1,1,13,52–55 and the activation energy for generation of monosaccharide was in the range 100–130 kJ mol−1.52,53 The activation energies for the hydrolysis of cellobiose and the generation of monosaccharide in this work are all relatively small, which indicates that the metallomicelle system displayed good catalytic efficiency for the breakage of β-glycoside bonds under mild conditions.
 | ||
Fig. 4 Plots of ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kcat vs. 1/T for the cellobiose hydrolysis and ln kcat vs. 1/T for the cellobiose hydrolysis and ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k2 vs. 1/T for the generation of monosaccharide. k2 vs. 1/T for the generation of monosaccharide. | ||
Further, the metallomicelle La(DMBO)2 was used to catalyze the hydrolysis of cellulose (Whatman 42 filter paper). The experimental results showed that for a reaction time of 20 h at pH 9.0 and 95 °C, the residual mass of insoluble filter paper was about 91.7%, and the yield and selectivity of total reducing sugar were 5.7% and 70%, respectively. This indicated that the metallomicelle La(DMBO)2 could catalyze the hydrolytic breakage of β-1,4-glucoside bond inside the cellulose molecule as does β-glucosidase.
4. Conclusions
This work investigated the catalytic hydrolysis of cellobiose to monosaccharide under mild conditions. The novel metallomicelle La(DMBO)2 displayed excellent catalytic activity for both the conversion of cellobiose and yield of monosaccharide (glucose, fructose and 1,6-anhydroglucose) in weakly alkaline aqueous solutions at relative low temperature (80–110 °C). This catalytic activity could be attributed to the special enzyme-like micelle microenvironment and the strong nucleophilicity of the N–OH functional group. The relatively low activation energy Ea1 = 96.13 kJ mol−1 for the conversion of cellobiose and Ea2 = 84.6 kJ mol−1 for the generation of monosaccharide was calculated. This work may provide a new technology and method for the hydrolysis of cellulose to monosaccharide under mild conditions.Acknowledgements
This work has been supported by the National Natural Science Foundation of China (no. 21273156).Notes and references
- V. Laurent, F. Markus, D. H. John, P. A. Martin and R. S. Kenneth, Green Chem., 2009, 11, 390 RSC.
- A. Fukuoka and P. L. Dhepe, Angew. Chem., Int. Ed., 2006, 45, 5161 CrossRef CAS PubMed.
- Z. Srokol, A. G. Bouche, A. Estrik and J. A. Peters, Carbohydr. Res., 2004, 339, 1717 CrossRef CAS PubMed.
- A. V. Bridgwater, Chem. Eng. J., 2003, 91, 87 CrossRef CAS.
- J. J. Wang, J. W. Ren, X. H. Liu, J. X. Xi, Q. E. Xia, Y. H. Zu, G. Z. Lu and Y. Q. Wang, Green Chem., 2012, 14, 2506 RSC.
- R. Vinu and L. J. Broadbelt, Energy Environ. Sci., 2012, 5, 9808 CAS.
- R. Wolfenden and Y. Yuan, J. Am. Chem. Soc., 2008, 130, 7548 CrossRef CAS PubMed.
- L. R. Lynd, M. S. Laser, D. Bransby, B. E. Dale, B. Davison, R. Hamilton, M. Himmel, M. Keller, J. D. McMillan, J. Sheehan and C. E. Wyman, Nat. Biotechnol., 2008, 26, 169 CrossRef CAS PubMed.
- G. SriBala and R. Vinu, Ind. Eng. Chem. Res., 2014, 53, 8714 CrossRef CAS.
- I. A. Malester, M. Green and G. Shelef, Ind. Eng. Chem. Res., 1992, 31, 1998 CrossRef CAS.
- R. J. Chimentao, E. Lorente, G. F. Gispert, F. Medina and F. Lopez, Carbohydr. Polym., 2014, 111, 116 CrossRef CAS PubMed.
- L. Shuai and X. J. Pan, Energy Environ. Sci., 2012, 5, 6889 CAS.
- S. Kumar and R. B. Gupta, Ind. Eng. Chem. Res., 2008, 47, 9321 CrossRef CAS.
- M. Sasaki, M. Furukawa, K. Minami, T. Adschiri and K. Arai, Ind. Eng. Chem. Res., 2002, 41, 6642 CrossRef CAS.
- T. L. Yong and Y. Matsumura, Ind. Eng. Chem. Res., 2013, 52, 5626 CrossRef CAS.
- Y. Yu, Z. M. Shafie and H. W. Wu, Ind. Eng. Chem. Res., 2013, 52, 17006 CrossRef CAS.
- K. L. Zhuo, Q. Z. Du, G. Y. Bai, C. Y. Wang, Y. J. Chen and J. J. Wang, Carbohydr. Polym., 2014, 115, 49 CrossRef PubMed.
- R. Wahlstrom, A. King, A. Parviainen, K. Kruus and A. Suurnakki, RSC Adv., 2013, 3, 20001 RSC.
- M. Liu, S. Y. Jia, Y. Y. Gong, C. S. Song and X. W. Guo, Ind. Eng. Chem. Res., 2013, 52, 8167 CrossRef CAS.
- Y. Hsuanyu and K. J. Laidler, Can. J. Biochem. Cell Biol., 1985, 63, 167 CAS.
- J. Bian, F. Peng, X. P. Peng, X. Xiao, P. Peng, F. Xu and R. C. Sun, Carbohydr. Polym., 2014, 100, 211 CrossRef CAS PubMed.
- S. Das, B. S. David, H. F. Ji, J. McDonough and Y. Wei, J. Mol. Catal. B: Enzym., 2011, 70, 49 CrossRef CAS PubMed.
- A. R. Joo, M. Jeya, K. M. Lee, K. M. Lee, H. J. Moon, Y. S. Kim and J. K. Lee, Process Biochem., 2010, 45, 851 CrossRef CAS PubMed.
- C. Lai, G. M. Zeng, D. L. Huang and M. H. Zhao, Anal. Methods, 2014, 6, 312 RSC.
- L. Verdoucq, J. Moriniere, D. R. Bevan, A. Esen, A. Vasella, B. Henrissat and M. Czjzek, J. Biol. Chem., 2004, 279, 31796 CrossRef CAS PubMed.
- H. T. Li, X. H. Xu, H. H. Chen, Y. Zhang, J. Xu, J. Wang and X. Y. Lu, Bioresour. Technol., 2013, 134, 51 CrossRef CAS PubMed.
- J. H. Wang, Q. Q. Hou, L. H. Dong, Y. J. Liu and C. B. Liu, J. Mol. Graphics Modell., 2011, 30, 148 CrossRef CAS PubMed.
- D. E. Koshland and S. S. Stein, J. Biol. Chem., 1954, 208, 139 CAS.
- S. H. Gellman, R. Petter and R. Breslow, J. Am. Chem. Soc., 1986, 108, 2388 CrossRef CAS PubMed.
- D. Kou, X. G. Meng, Y. Liu, J. Du, X. M. Kou and X. C. Zeng, Colloids Surf., A, 2008, 324, 189 CrossRef CAS PubMed.
- S. Bhattacharya and N. Kumari, Coord. Chem. Rev., 2009, 253, 2133 CrossRef CAS PubMed.
- P. R. Sharma, P. R. Rajamohanan and A. J. Varma, Carbohydr. Polym., 2014, 113, 615 CrossRef CAS PubMed.
- M. D. Pluth, R. G. Bergman and K. N. Raymoda, Acc. Chem. Res., 2009, 42, 1650 CrossRef CAS PubMed.
- Y. Ye, Q. P. Ding and J. Wu, Tetrahedron, 2008, 64, 1378 CrossRef CAS PubMed.
- M. Boudou, C. Ogawa and S. Kobayashi, Adv. Synth. Catal., 2006, 348, 2585 CrossRef CAS.
- X. L. Li, H. Li, G. Q. Liu, Z. W. Deng, S. L. Wu, P. H. Li, Z. S. Xu, H. B. Xu and P. K. Chu, Biomaterials, 2012, 33, 3013 CrossRef CAS PubMed.
- M. Sasidharan, N. Gunawardhana, H. N. Luitel, T. Yokoi, M. Inoue, S. Yusa, T. Watari, M. Yoshio, T. Tatsumi and K. Nakashima, J. Colloid Interface Sci., 2012, 370, 51 CrossRef CAS PubMed.
- G. R. Whittell, M. D. Hager, U. S. Schubert and I. Manners, Nat. Mater., 2011, 10, 176 CrossRef CAS PubMed.
- R. Dobrawa and F. Wurthner, J. Polym. Sci., Part A: Polym. Chem., 2005, 43, 4981 CrossRef CAS.
- M. X. Cheng, T. Shi, H. Y. Guan, S. T. Wang, X. H. Wang and Z. Q. Jiang, Appl. Catal., B, 2011, 107, 104 CrossRef CAS PubMed.
- Y. Zhang, J. L. Xu, W. Qi, Z. H. Yuan, X. S. Zhuang, Y. Liu and M. C. He, Appl. Biochem. Biotechnol., 2012, 168, 144 CrossRef CAS PubMed.
- A. Fabian, G. Daza and A. D. Mackie, J. Phys. Chem. Lett., 2014, 5, 2027 CrossRef.
- T. Fujita, Y. Inaba, K. Ogino and W. Tagaki, Bull. Chem. Soc. Jpn., 1988, 61, 1661 CrossRef CAS.
- H. Kobayashi, M. Yabushita, T. Komanoya, K. Hara, I. Fujita and A. Fukuoka, ACS Catal., 2013, 3, 581 CrossRef CAS.
- V. Bravo, M. P. Paez, M. Aoulad and A. Reyes, Enzyme Microb. Technol., 2000, 26, 614 CrossRef CAS.
- A. M. J. Kootstra, H. H. Beeftink, E. L. Scott and J. P. M. Sanders, Biochem. Eng. J., 2009, 46, 126 CrossRef CAS PubMed.
- A. M. J. Kootstra, N. S. Mosier, E. L. Scott and H. H. Beeftink, Biochem. Eng. J., 2009, 43, 92 CrossRef CAS PubMed.
- J. W. Lee, R. C. L. B. Rodrigues, H. J. Kim, I. G. Choi and T. W. Jeffries, Bioresour. Technol., 2010, 101, 4379 CrossRef CAS PubMed.
- Y. Liu, X. G. Meng, W. F. Yu, X. H. Li and X. Peng, Acta Phys.-Chim. Sin., 2013, 29, 2263 CAS.
- L. Shuai, Q. Yang, J. Y. Zhu, F. C. Lu, P. J. Weimer, J. Ralph and X. J. Pan, Bioresour. Technol., 2010, 101, 3106 CrossRef CAS PubMed.
- C. A. Schall, A. P. Dadi and S. Varanasi, Biotechnol. Bioeng., 2006, 95, 904 CrossRef PubMed.
- B. M. Kabyemela, M. Takigawa, T. Adschiri, R. M. Malaluan and K. Arai, Ind. Eng. Chem. Res., 1998, 37, 357 CrossRef CAS.
- L. Negahdar, J. U. Oltmanns, S. Palkovits and R. Palkovits, Appl. Catal., B, 2014, 147, 677 CrossRef CAS PubMed.
- J. A. Bootsma and B. H. Shanks, Appl. Catal., A, 2007, 327, 44 CrossRef CAS PubMed.
- X. Liang, A. Montoya and B. S. Haynes, J. Phys. Chem. B, 2011, 115, 10682 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra14521f |
| This journal is © The Royal Society of Chemistry 2015 |