Photocatalytic activity of hierarchically structured, thermally stable, anatase particles†
Andrijana Sever Škapina,
Luka Škrlepa,
Danilo Suvorovb,
Vojka Žuničb and
Srečo D. Škapin*b
aCivil Engineering Institute, Slovenian National Building, Dimičeva 12, 1000 Ljubljana, Slovenia
bJožef Stefan Institute, Jamova 39, 1000 Ljubljana, Slovenia. E-mail: Sreco.Skapin@ijs.si; Fax: +386 1 4773875; Tel: +386 1 4773708
First published on 9th March 2015
Abstract
In order to avoid the potential health problems associated with nanosized particles, solvothermal synthesis was employed for the preparation of doped and undoped, hierarchically structured, spherical anatase, ranging in size from 2 to 6.4 μm. The resulting particles showed a mesoporous microstructure and, consequently, a high specific surface area of up to 208 m2 g−1. A detailed SEM analysis confirmed the hierarchical structure of the spheres, consisting of subunits with a size from 10 to 21 nm, depending on the starting composition. For the thermal stabilization of the anatase phase and to slow down the growth of the nanosize particles during heating, various dopants were added to the anatase. As a result, anatase codoped with Ce, Si and Zr exhibited a high thermal stability up to 1000 °C, compared to 700 °C for the undoped anatase. The photocatalytic activities of the synthesized solids were quantitatively evaluated by monitoring the rate of degradation for isopropanol in a gas phase reactor system. Among the samples that were heat treated at 500 °C, the highest photocatalytic activity was exhibited by the Ce-doped sample. In the case of the samples which were heat treated at 1050 °C, the undoped and Ce-doped samples were inactive under UV light, while the samples doped with Ce, Zr and Si exhibited considerable photocatalytic activity.
1. Introduction
Titanium dioxide exhibits many useful optical, electrical and photocatalytic properties, depending on the particle size, the phase composition, the microstructure and the chemical composition. Control of these properties is vital for the desired performance of these materials in applications like sensors, photocatalysis, electronics and pigments.1 There are reports of photocatalytic activity for all three titania polymorphs, i.e. anatase, rutile and brookite, and their various mixtures.2–4 However, anatase and mixtures of anatase and rutile are the most commonly used in photocatalytic applications.1,2 The photocatalytic activity also depends on the specific surface area and the degree of crystallinity. Heterogeneous photocatalytic reactions depend on the energy source, the reactant adsorption, the catalyst activity, the reaction mechanism and the product desorption. All these parameters may influence the ideal properties of the catalytic material for a particular reaction.1 The chemical composition of the nanosized anatase can have an influence on the particle formation during the synthesis as well as on its morphology, surface acidity and band gap.Upon heating, the anatase and brookite polymorphs of titanium dioxide eventually transform into the thermodynamically stable rutile form. This usually means a loss of photocatalytic activity, compared to that of the anatase.4 The transition temperature is important for certain applications where high temperatures are used, like tile or roof-tile production. A mechanism for this phase transition has already been proposed by several authors.5 For the anatase-to-rutile transformation Reidy et al.6 suggested a so-called “critical size mechanism”. Anatase particles are supposed to grow with increasing temperature; then after reaching a critical size they transform into the rutile phase. In general, the phase-transition temperature of many systems can be controlled by doping. Reidy et al.7 used silicon and zirconium doping to inhibit the material transport and particle growth during heat treatments. There are also reports of doping with cerium,8–10 silicon,11,12 sulfur,13 and phosphorus,14 to increase the thermal stability of the anatase. It has been shown that the mesoporous anatase particles as well as nanotube arrays are stable to much higher temperatures than non-mesoporous particles.15–18 Moreover, Grover et al.19 reported that rice-like anatase could be stable up to 800 °C, however, the particles completely altered their morphology during such thermal treatment. Additionally, in anatase–silica composites the anatase phase is stable at higher temperatures.20–22
Anatase nanoparticles can be synthesized by different methods.1 The solvothermal, non-aqueous method yields materials with several very interesting features, for example, good crystallinity of the products at moderate reaction temperatures. This method is less sensitive to reaction conditions than the syntheses based on aqueous systems.23 Garnweitner et al.24 synthesized anatase nanoparticles from titanium isopropoxide in different aldehydes and ketones used as solvents. Reactions involving a slow aldol condensation with an elimination of the water result in anatase particles with excellent crystallinity. In our investigation we applied the aforementioned method to synthesize the anatase-based nanoparticles, using acetone as the solvent reactant.
The main goals of our investigation were to prepare materials that would (i) exhibit photocatalytic activity even after a heat treatment at very high temperatures, at least 1050 °C, and (ii) possess non-harmful characteristics. However, it is well known that after a high-temperature treatment, due to the transformation of the anatase into rutile, sintering and particle growth, most of the existing nano-titania tends to lose its photocatalytic activity. The doping shifts the transformation to higher temperatures, whereas the sintering is significantly suppressed. On the other hand, the nanosized TiO2 particles (<100 nm) are known to have harmful effects on the cell membrane, and even for non-toxic titania, scientists warn that extreme caution must be used when working with such particles.25–29
A compromise was achieved by preparing micron-sized aggregates showing a hierarchically nanostructured morphology, which nevertheless retain their nanoparticulate nature (high surface area, porosity, etc.) and thus their inherent photocatalytic activity. The influence of the type and the amount of dopant used on the physicochemical properties of anatase is also reported.
2. Experimental section
Preparation of TiO2-based photocatalysts
Reagent-grade metal alkoxides titanium isopropoxide (Ti(OiPr)4), zirconium-n-butoxide (Zr(OBu)4), tetraethoxysilane (Si(OEt)4) and cerium(III) nitrate hexahydrate (Ce(NO3)3·6H2O), obtained from Aldrich, were used as received without further purification. In a typical experiment the raw materials, Ti(OiPr)4, Zr(OBu)4, Si(OEt)4 and Ce(NO3)3, were dissolved in 1 mol of acetone in different mole ratios (Table 1). The prepared starting solution was put into a 100 mL autoclave with a Teflon liner. The autoclave was heated in an oven at 140 °C for 24 h and then left to cool. The obtained yellow-to-amber suspension was filtered and washed with acetone. The filtered product was dried at 110 °C for 5 h. To remove all the residual organic species and volatiles the solids were heated at a rate of 5 °C min−1 to 500 °C and left at this temperature for 2 h. For a determination of the temperature of the anatase-to-rutile transformation the samples were additionally heated from 550 °C to 1050 °C in 50 °C intervals and then soaked at each temperature for 0.5 h. The heating rate for these firing experiments was set to 5 °C min−1.| Sample ID | Dissolved moles of the reactants into 1 mol of acetone | Average aggregate size [μm] | Primary particle size [nm] | BET specific surface area [m2 g−1] | ||||
|---|---|---|---|---|---|---|---|---|
| Ti(OiPr)4 | Si(OEt)4 | Zr(OBu)4 | Ce(NO3)3 | 500 °C | 1050 °C | |||
| T | 0.1000 | 0 | 0 | 0 | 5.6 ± 2.3 | 21 | 65 | n.a. |
| C | 0.1000 | 0 | 0 | 0.0001 | 6.4 ± 2.5 | 16 | 55 | n.a. |
| SZ1 | 0.0890 | 0.0084 | 0.0026 | 0.0001 | 4.7 ± 2.9 | 14 | 152 | 0.7 |
| SZ2 | 0.0861 | 0.0086 | 0.0053 | 0.0001 | 3.4 ± 1.9 | 14 | 158 | 6.5 |
| SZ3 | 0.0812 | 0.0163 | 0.0025 | 0.0001 | 2.8 ± 1.4 | 11 | 194 | 4.3 |
| SZ4 | 0.0782 | 0.0167 | 0.0051 | 0.0001 | 2.0 ± 1.2 | 10 | 208 | 14.5 |
Materials characterization
The morphology and the size of the particles were examined with a field-emission scanning electron microscope (FE-SEM, Supra 35 VP, Carl Zeiss) equipped with an energy-dispersive spectrometer (EDS, Inca 400, Oxford Instruments). The particle size of the spherical aggregates was obtained from FE-SEM micrographs by applying the software Image Tool for Windows. Phase identification was based on X-ray powder diffraction using a D4 Endeavor, Bruker AXS diffractometer with Cu Kα radiation (λ = 0.15406 nm) and a Sol-X energy-dispersive detector within the angular range 2Θ from 10 to 70 degrees, a step size of 0.02 degrees and a collection time of 5 seconds. The mean crystallite size of the anatase primary particles was determined from the (101) X-ray diffraction peak using the Scherrer equation d = Kλ/β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Θ, where a value K = 0.9 was used for the shape factor. The surface area of the powders was measured using a BET surface area analyzer (Micromeritics ASAP 2020). The internal microstructures of the prepared spherical, micron-sized, anatase particles were analyzed using a JEOL JEM 2010F transmission electron microscope (TEM) operating at 200 kV. The XPS analyses were performed with a TFA XPS spectrometer (Physical Electronics Inc.). The sample surfaces were excited by X-ray radiation from a monochromatic Al source with an energy of 1486.6 eV. The high-energy-resolution spectra were acquired with an energy analyzer operating at a resolution of about 1.0 eV.
Θ, where a value K = 0.9 was used for the shape factor. The surface area of the powders was measured using a BET surface area analyzer (Micromeritics ASAP 2020). The internal microstructures of the prepared spherical, micron-sized, anatase particles were analyzed using a JEOL JEM 2010F transmission electron microscope (TEM) operating at 200 kV. The XPS analyses were performed with a TFA XPS spectrometer (Physical Electronics Inc.). The sample surfaces were excited by X-ray radiation from a monochromatic Al source with an energy of 1486.6 eV. The high-energy-resolution spectra were acquired with an energy analyzer operating at a resolution of about 1.0 eV.
For the characterization of the optical properties a UV-vis-NIR spectrophotometer (Shimadzu UV-3600) equipped with an integrating sphere (ISR-3100, 60 mm) was used. The diffuse reflectance spectra were recorded in the range 200–800 nm under ambient conditions. The band-gap energy for the samples was calculated based on experimentally obtained diffuse reflectance spectra using computer software supplied by Shimadzu.
Photocatalytic study
The photocatalytic activity of the samples was measured by monitoring the degradation of a model organic compound, isopropanol, as well as the acetone formation in a gas medium utilizing FT-IR spectroscopy. Under UV irradiation, the isopropanol, in contact with the photocatalytically active solid substance, oxidizes into acetone and then into several further degradation products. The method enables a quantitative determination of the photocatalytic activity and is described in detail elsewhere.30,31 The light source was a 300 W xenon lamp (Newport Oriel Instrument, USA) with a light intensity of approximately 25 W m−2 in the λ range 320–400 nm. The spectral power distribution of the light source (Fig. S1†) was evaluated by applying a USB2000+ Miniature Fiber Optic Spectrometer (Ocean Optics). It was measured 6 cm from the light source, at the same area as the samples were placed during the UV exposure. The temperature and relative humidity were set to 23 ± 2 °C and 25 ± 5%, respectively. For all the measurements the same quantity of sample, 50 mg, was examined and the same initial quantity of liquid isopropanol (3 μl) was injected into the sealed reacting system through a septum. The initial concentration of isopropanol in the sealed reactor was 700 ppm in a gas phase. The photocatalytic activity of the prepared samples was evaluated as the rate constant of the initial acetone formation. The photocatalytic oxidation of isopropanol to form acetone is rapid at room temperature, while the following oxidation of acetone to further degradation products is slower.32,33 A kinetic model for the calculation of the acetone formation-rate constants has been described in detail elsewhere.31 The accuracy of the method was estimated to be 1 ppm h−1.3. Results and discussion
A low-temperature solvothermal synthesis was used in the preparation of anatase-based particles from reaction solutions, with the starting reactant concentrations as summarized in Table 1. Details about the synthesis procedure and the proposed reaction mechanism for anatase formation are described elsewhere.23,24 Basically, the aldol condensation of acetone takes place, with elimination of water that reacts with titanium isopropoxide forming Ti–O–Ti bonds and isopropanol. Further condensation leads to the formation of oxide nanoparticles. For the purpose of our investigation the doped anatase was prepared by the addition of Ce(NO3)3·6H2O and metal alkoxides Si(OEt)4 and Zr(OBu)4 to the starting solution in different ratios. It was expected that a simultaneous hydrolysation of the present alkoxides took place, resulting in Ti–O–M (M = Ti, Si or Zr) bonds under the applied reaction conditions (Fig. S2†).Fig. 1 shows the XRD patterns of the as-prepared TiO2-based powders. All the diffraction peaks were matched to the anatase phase (JCPDS card no. 21-1272) and there were no additional peaks corresponding to secondary phases. Single phase nature of the as-prepared TiO2-based samples was also confirmed by Raman spectroscopy (Fig. S3 and S4†). The degree of crystallinity of the non-doped TiO2 is quite remarkable for such a low synthesis temperature (Fig. 1, pattern (a)). Similar observations have been reported by other authors.24 The sample with the cerium addition also shows a similar crystallinity (Fig. 1, pattern (b)). However, the samples with added Si and Zr in different ratios exhibit diffractograms with broadened XRD peaks, lower intensity and a slightly higher background, suggesting the presence of finer and less-well-crystallized particles (Fig. 1, patterns (c)–(f)), due to the incorporation of dopants into the Ti–O network (Fig. S5 in the ESI†).
 | ||
| Fig. 1 XRD patterns of the as-prepared TiO2-based solids: (a) sample T (pure TiO2), (b) sample C, (c) sample SZ1, (d) sample SZ2, (e) sample SZ3 and (f) sample SZ4. | ||
The as-prepared samples which were additionally calcined for 2 h at 500 °C exhibit similar XRD patterns to those of the samples prior to calcinations. These results suggest that the nanostructure of the aggregated spherical particles containing the primary nanoparticles and the sample's crystallinity did not change during calcinations.
The information about the particle size and morphology was obtained from a detailed SEM investigation of the prepared solids. Fig. 2 shows a series of FE-SEM micrographs of selected TiO2-based solids, each sample is presented at two different magnifications. Evidently, all the samples appeared as hierarchically structured spherical clusters. The size of these nearly perfect spheres is summarized in Table 1. The average size of the spherical aggregates for pure anatase (sample T) is determined to be approximately 5.6 μm, whereas for sample C the size increases up to 6.4 μm. However, the opposite trend in the aggregate size can be observed at higher levels of doping. Thus, the highest doped anatase, sample SZ4, forms aggregates with a size of approximately 2.0 μm in diameter (Table 1, Fig. 2a, c, e and g). Higher magnifications of SEM images clearly reveal that the spherical aggregates are mesoporous and that they consist of smaller subunits. A mesoporous assembly of the spherical aggregates was confirmed by SSA measurements, which showed that the average pore size was 3–5 nm. The pure anatase-type TiO2 exhibits individual nanocrystallites with a mean size of 21 nm (Fig. 2b); however, the doped samples have larger, nearly spherical subunits, up to approximately 70 nm in diameter, which are composed of even smaller, densely packed, primary nanocrystallites of a size up to 11 nm (Table 1, Fig. 2d, f and h). Obviously, the crystallite size decreases significantly with dopant content. However, upon adding ZrO2 to anatase, the opposite effect was reported. Specifically, pure anatase and anatase doped with 10 mol% of ZrO2 formed 9 nm- and 13 nm-sized particles, respectively.34 Finally, according to the literature data, SiO2 and Ce2O3 additions have no significant effect on the size of anatase-type crystallites.21,35
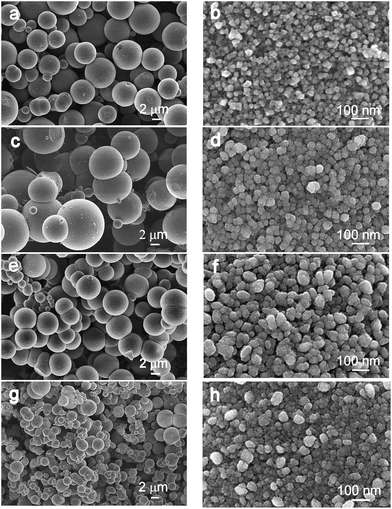 | ||
| Fig. 2 FE-SEM micrographs of the samples heat treated at 500 °C: (a) and (b) sample T, (c) and (d) sample C, (e) and (f) sample SZ3, and (g) and (h) sample SZ4. | ||
The specific surface area of pure TiO2 is 65 m2 g−1 and this value decreases with the addition of 0.1% Ce to 55 m2 g−1 (sample C); however, larger amounts of dopants result in proportionally more porous aggregates, and thus, the sample SZ4 shows the highest SSA value of 208 m2 g−1 (Table 1).
Microspherical-doped TiO2-based structures were further analyzed using EDS analyses. At least seven measurements on different spheres were carried out for each sample. The analysis of pure TiO2 and TiO2 with 0.1% of Ce addition shows only the presence of Ti and O (Fig. 3, spectra (a)). The amount of Ce in the sample C is too low to be qualitatively identified by EDS. The results of the analysis of highly doped TiO2 are slightly scattered, since the shapes of the analyzed spherical objects were far from ideal for such an analysis. Nevertheless, in the codoped samples Ti cations along with Si and Zr remain in similar proportions as used when preparing the initial mixture (Fig. 3, spectra (b) and (c)).
 | ||
| Fig. 3 Characteristic EDS spectra with a qualitative elemental analysis of TiO2-based microspheres for the selected samples: (a) C, (b) SZ3 and (c) SZ4. | ||
The results shown above suggest that dopants may either enter into the anatase crystal structure or are concentrated on the surface of the primary anatase crystallites. The possibility that dopants may have precipitated as discrete phases was also examined by routine X-ray mapping EDS analysis on many locations of the analyzed samples, but the final results did not confirm the formation of discrete phases such as SiO2 or ZrO2.
XPS was used to study the chemical state of the dopants Si and Zr on the surface of the samples calcined at 500 °C, whereas the amount of added Ce is too small to be detected. Fig. S5a† shows the Si 2p spectra from the sample SZ4, where a single peak corresponding to the bonding energy of Si at 102.1 eV is present. Since the typical bonding energy of Si in SiO2 is around 104 eV, we can conclude that Si forms Si–O–Ti bonds. The spectra of Zr 3d in Fig. S5b† shows a peak with a binding energy at 182.3 eV that can be assigned to the Zr4+ valence state in the ZrO2.
Based on lattice-parameter measurements, many authors have proposed that a certain amount of Si4+ may incorporate into the anatase crystal structure.22,36,37 In contrast, Hirano et al.21 state that SiO2 and anatase form a TiO2/SiO2 composite, where SiO2 coexists as an amorphous phase over the anatase crystalline nanoparticles. Similarly, it has been proposed that the CeO2−x that co-precipitates with anatase does not incorporate into the anatase crystal structure due to the much larger ionic radius of Ce3+ and Ce4+ (rCe3+ = 0.103 nm and rCe4+ = 0.093 nm) compared to that of Ti4+ (rTi4+ = 0.068 nm), thus forming a surrounding amorphous phase.8,35 In the anatase–ZrO2 system, up to approximately 40 mol% of TiO2 can be substituted by Zr and a monoclinic ZrO2 as a secondary phase is detected at a content of 50 mol% ZrO2,34 however Zhang et al.38 reported that a ZrTiO4 as a secondary phase was detected at 20 mol% ZrO2. Our measurements of the anatase lattice parameters a and c of the heated samples at 500 °C show a slight variation of the parameters with increasing doping level, where a varies between 0.378 and 0.379 nm and c within 0.953 and 0.951 nm.
TEM analyses were applied in order to observe the internal structure of the micron-sized anatase particles. Since the as-prepared particles were not suitable for a TEM analysis due to their large size (Fig. S6†), they were mixed with epoxy resin and applied between two Si wafers and further treated as a bulk sample by thinning, dimpling and ion milling. TEM micrographs of the particles revealed that the micron-sized anatase particles consisted of nanosized, primary crystallites that are partially surrounded by pores and thus exhibited an open mesoporosity (Fig. 4). The results support the conclusions based on the SEM and BET analyses.
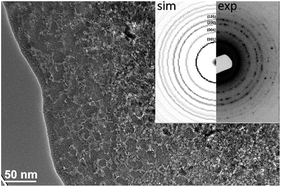 | ||
| Fig. 4 Bright-field TEM image and corresponding SAED pattern of the sample SZ4 (thin layer on the surface of the particle is the epoxy resin). | ||
The transformation of anatase to rutile (A–R) was reported to be an irreversible first-order process due to the metastability of the anatase structure and is associated with a volume shrinkage of approximately 8%. The transformation temperature, reported to be higher than 400 °C, depends on several factors, such as the presence of foreign ions, the atmosphere, the method of synthesis, the thermal treatment, the sample form (bulk, film), the grain size, etc.5,21,39,40 Since the A–R transformation requires relatively high energies for nucleation and further cooperative movement it occurs at relatively high temperatures. A side effect of the process is the simultaneous sintering of the primary TiO2 particles. The A–R transformation in nanocrystalline solids is proposed as a two-step process, consisting of: (i) particle agglomeration by grain-boundary diffusion until the critical particle size is reached for transformation, and (ii) a spontaneous A–R transformation.7 In order to determine the thermal stability of the synthesized anatase, the samples were thermally treated at different temperatures from 550 °C to 1050 °C, with a treatment period of 0.5 h at each temperature. The relative amount of the phases present in the fired samples was calculated using the Spurr equation:41
| xA (wt%) = 100/(1 + 1.265(IR/IA)), | (1) |
 | ||
| Fig. 5 XRD patterns of heat-treated samples: (a) T (pure anatase) at different temperatures (700 °C, 800 °C and 850 °C) and (b) T (800 °C), C (950 °C) and SZ1, SZ2, SZ3 and SZ4 (1050 °C). | ||
| Samples | ||||||
|---|---|---|---|---|---|---|
| T | C | SZ1 | SZ2 | SZ3 | SZ4 | |
| T [°C] | 800 | 950 | 1050 | 1050 | 1050 | 1050 |
| xA [wt%] | 25 | 84 | 82 | 78 | 91 | 71 |
| dA [nm] | 71 | 60 | 29 | 28 | 24 | 23 |
The samples doped with various dopant amounts show a higher thermal stability, up to 1000 °C. Firing at 1050 °C results in a partial anatase transformation into rutile, whereby the amount transformed depends on the starting composition (Table 2). The lowest amount of rutile phase (9%) was found in the sample SZ3. It should be stressed that the XRD pattern showed no secondary phases, even when larger amounts of Si- and Zr-based dopants were added to the starting compositions, suggesting that the dopants entered into the anatase crystal structure.
The mean size of the primary anatase particles, as determined by Scherrer's equation in the heat-treated samples exhibiting rutile as a secondary phase, decreases from 71 nm for pure TiO2 (sample T) to 23 nm for the sample SZ4 (Table 2).
Fig. 6 and 7 show micrographs of the fired samples in which a partial and/or complete A–R transformation has occurred. It is clear that after the transformation the TiO2 retains the spherical shape of the particles. Furthermore, a detailed SEM investigation of the samples revealed that the transformation takes place through the bulk of an individual spherical particle, accompanied by the growth and sintering of the primary anatase particles. Thus, on the surface of the samples T and C, fired at 800 °C and 950 °C, respectively, anatase particles/grains with an estimated average size ranging from 25 to 150 nm are observed (Fig. 6b and 7b). It is obvious that the primary particles are partially sintered, forming a non-porous spherical body. However, higher-level doped TiO2 (samples SZ1–4), heated at 1050 °C for 0.5 h, still possesses a partially mesoporous structure, although the primary particles are grown from 15 nm to 100 nm and these subunits are partially bridged (Fig. 7d and f). The size of the subunits in the heated samples, determined from the XRD patterns using Scherrer's equation (Table 2), fits within these, rather wide, size distributions. This observation is in agreement with the recently proposed two-step mechanism of the A–R transformation, consisting of anatase particle growth and a transformation process of primary anatase nanocrystallites. The samples fired above the transformation temperature in which only the rutile phase was detected, appeared as spherical particles with a mostly smooth surface. These spheres are built up of larger rutile grains with well-developed grain boundaries. The size of the rutile grains depends on the size of spheres and may range from few hundreds of nm to a few μm (Fig. 6c). The BET measurements of the co-doped samples heated at 1050 °C (Table 1) show an increase in the specific surface area by increasing the amount of dopants and thus confirming the SEM results, i.e., that the heavily doped anatase still exhibits a partially open porosity. The band-gap energies of the prepared photocatalysts were determined from the diffuse reflectance spectra (Fig. S7†) using the Kubelka–Munk transformation (Table S1†). The obtained results show that the band-gap values for the samples are around 3.2 eV and that the dopants have a minor influence on the size of the band gap.
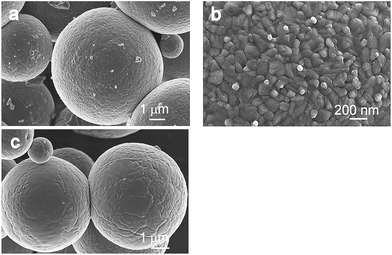 | ||
| Fig. 6 FE-SEM micrographs of the sample T heat treated for 0.5 h at: (a) and (b) 800 °C, containing 75% of rutile and (c) 850 °C, consisting of pure rutile. | ||
 | ||
| Fig. 7 FE-SEM micrographs of the samples heat treated at high temperatures for 0.5 h: (a) and (b) C at 950 °C, (c) and (d) SZ2 at 1050 °C, and (e) and (f) SZ3 at 1050 °C. | ||
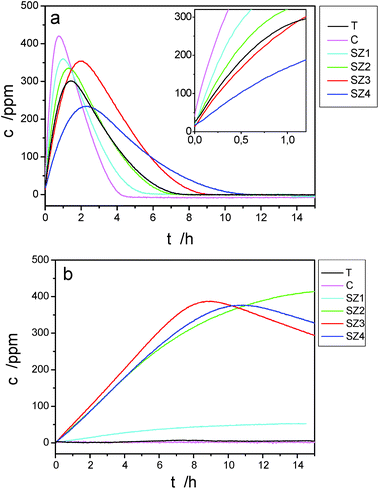
The samples were further analyzed with regard to their photocatalytic activity. The latter was measured by monitoring the initial acetone formation during the photocatalytic degradation of isopropanol in the presence of the samples and UV light. The photocatalytic-activity results of the doped and non-doped samples are shown in Table 3 and in Fig. 8a and b. The formation of acetone during the photocatalytic degradation of isopropanol in the presence of samples heat treated at 500 °C is illustrated in Fig. 8a, while the formation of acetone in the presence of samples that were additionally heated from 550 °C to 1050 °C in 50 °C intervals, maintaining each temperature for 0.5 h, is shown in Fig. 8b. In all the photocatalytic measurements at the beginning of the experiment the UV lamp was switched off to achieve an adsorption–desorption equilibrium for the injected isopropanol. The time zero in both figures represents the point when the UV lamp was switched on. The photocatalytic activity of the powders was assessed according to the value of the rate constant representing the oxidation of isopropanol into acetone. This reaction is usually considered a zero-order reaction, while the further oxidation can be assumed to be a first-order reaction.30,31
| Sample ID | Rate constant of the initial acetone formation [ppm h−1] | |
|---|---|---|
| Samples heated at 500 °C | Samples additionally heated at 1050 °C | |
| T | 370 | 0 |
| C | 907 | 0 |
| SZ1 | 679 | 10 |
| SZ2 | 477 | 47 |
| SZ3 | 364 | 54 |
| SZ4 | 174 | 45 |
In the present context we are merely interested in the relative change of the rate constant of the isopropanol oxidation into acetone, which is a measure of the photocatalytic activity. The results for different samples are summarized in Table 3. It is clear that the heat treatment of the doped and non-doped samples at 500 °C results in very good activity. The activity of the non-doped TiO2 was found to be 370 ppm h−1, which is comparable with the value of the photocatalytic activity (337 ppm h−1) of the reference sample Hombikat UV 100 (anatase nanopowder), which was presented in a previous paper.42 The doping with Ce increases the activity by almost three times, while the activity is increased in a similar way for samples SZ1 and SZ2. For sample SZ3 the activity is approximately the same as for the non-doped TiO2, while in the case of sample SZ4 the activity is slightly decreased. However, the photocatalytic activity of the samples, when additionally treated at 1050 °C, is rather different. The samples T and C, when treated at 1050 °C, show basically no photocatalytic activity. On the other hand, all the doped samples, SZ1, SZ2, SZ3 and SZ4, retain some activity (from 10 to 54 ppm h−1) even after being heat treated at 1050 °C. This is consistent with the results of the morphological and structural investigations. For example, the highest activity after heat treatment at 1050 °C (54 ppm h−1) is observed in the sample SZ3, the one with the largest amount of untransformed anatase.
4. Conclusions
Micrometer-sized, spherical, mesoporous particles of un-doped and doped anatase were successfully prepared by the solvothermal synthesis method in acetone media. Hierarchically assembled, spherical particles from nanosized primary crystallites were formed under certain experimental conditions in the absence of templating agents. Heat-treatment experiments conducted on the prepared solids confirmed that the used dopants increase the temperature of the anatase-to-rutile transformation. Thus, the pure anatase started to transform into rutile above 700 °C; the anatase with the addition of 0.1 mol% Ce, above 900 °C; while the anatase co-doped with Si and Zr is stable up to 1000 °C. The smallest amount of transformed anatase at 1050 °C was found for sample SZ3, containing 16.3 mol% Si, 2.5 mol% Zr and 0.1 mol% Ce.Acknowledgements
The authors gratefully acknowledge Dr Janez Zavašnik and Ms Andreja Šestan BSc. for their help in performing the TEM analysis, and Dr Janez Kovač for the XPS analysis.References
- X. Chen and S. S. Mao, Chem. Rev., 2007, 107, 2891–2959 CrossRef CAS PubMed.
- S. Yin, Y. Aita, M. Komatsu and T. Sato, J. Eur. Ceram. Soc., 2006, 26, 2735–2742 CrossRef CAS PubMed.
- S. Yin, K. Ihara, M. Komatsu, Q. Zhang, F. Saito, T. Kyotani and T. Sato, Solid State Commun., 2006, 137, 132–137 CrossRef CAS PubMed.
- S. Bakardjieva, J. Šubrt, V. Štengl, M. J. Dianez and M. J. Sayagues, Appl. Catal., B, 2005, 58, 193–202 CrossRef CAS PubMed.
- R. D. Shannon and J. A. Pask, J. Am. Ceram. Soc., 1965, 48, 391–398 CrossRef CAS PubMed.
- D. J. Reidy, J. D. Holmes and M. A. Morris, J. Eur. Ceram. Soc., 2006, 26, 1527–1534 CrossRef CAS PubMed.
- D. J. Reidy, J. D. Holmes and M. A. Morris, Ceram. Int., 2006, 32, 235–239 CrossRef CAS PubMed.
- Y.-H. Xu, H.-R. Chen, Z.-X. Zeng and B. Lei, Appl. Surf. Sci., 2006, 252, 8565–8570 CrossRef CAS PubMed.
- Q.-Z. Yan, X.-T. Su, Z.-Y. Huang and C.-C. Ge, J. Eur. Ceram. Soc., 2006, 26, 915–921 CrossRef CAS PubMed.
- R. F. de Farias and C. Airoldi, J. Non-Cryst. Solids, 2005, 351, 84–88 CrossRef CAS PubMed.
- H. Kominami, M. Kohno, Y. Matsunaga and Y. Kera, J. Am. Ceram. Soc., 2001, 84, 1178–1180 CrossRef CAS PubMed.
- C. He, B. Tian and J. Zhang, J. Colloid Interface Sci., 2010, 344, 382–389 CrossRef CAS PubMed.
- E. M. Rockafellow, L. K. Stewart and W. S. Jenks, Appl. Catal., B, 2009, 91, 554–562 CrossRef CAS PubMed.
- L. Körösi, S. Papp, I. Bertóti and I. Dékány, Chem. Mater., 2007, 19, 4811–4819 CrossRef.
- D. J. Reidy, J. D. Holmes, C. Nagle and M. A. Morris, J. Mater. Chem., 2005, 15, 3494–3500 RSC.
- K. Charette, J. Zhu, S. O. Salley, K. Y. S. Ng and D. Deng, RSC Adv., 2014, 4, 2557–2562 RSC.
- D.-W. Lee, S.-J. Park, S.-K. Ihm and K.-H. Lee, Chem. Mater., 2007, 19, 937–941 CrossRef CAS.
- B. M. Rao and S. C. Roy, RSC Adv., 2014, 4, 38133–38139 RSC.
- I. S. Grover, S. Singh and B. Pal, RSC Adv., 2014, 4, 24704–24709 RSC.
- D.-W. Lee, S.-K. Ihm and K.-H. Lee, Chem. Mater., 2005, 17, 4461–4467 CrossRef CAS.
- M. Hirano, K. Ota and H. Iwata, Chem. Mater., 2004, 16, 3725–3732 CrossRef CAS.
- D. M. Tobaldi, A. Tucci, A. S. Škapin and L. Esposito, J. Eur. Ceram. Soc., 2010, 30, 2481–2490 CrossRef CAS PubMed.
- M. Niederberger, G. Garnweitner, N. Pinna and G. Neri, Prog. Solid State Chem., 2005, 33, 59–70 CrossRef CAS PubMed.
- G. Garnweitner, M. Antonietti and M. Niederberger, Chem. Commun., 2005, 3, 397–399 RSC.
- Y. Nakagawa, S. Wakuri, K. Sakamoto and N. Tanaka, Mutat. Res., 1997, 394, 125–132 CAS.
- P. Amézaga-Madrid, R. Silveyra-Morales, L. Córdoba-Fierro, G. V. Nevárez-Moorillón, M. Miki-Yoshida, E. Orrantia-Borunda and F. J. Solis, J. Photochem. Photobiol., B, 2003, 70, 45–50 CrossRef.
- J. Wang, G. Zhou, C. Chen, H. Yu, T. Wang, Y. Ma, G. Jia, Y. Gao, B. Li, J. Sun, Y. Li, F. Jiao, Y. Zhao and Z. Chai, Toxicol. Lett., 2007, 168, 176–185 CrossRef CAS PubMed.
- V. H. Grassian, P. T. O'Shaughnessy, A. Adamcakova-Dodd, J. M. Pettibone and P. S. Thorne, Environ. Health Perspect., 2007, 115, 397–402 CrossRef CAS PubMed.
- F. Pacheco-Torgal and S. Jalali, Construct. Build. Mater., 2011, 25, 582–590 CrossRef PubMed.
- T. Marolt, A. Sever Škapin, J. Bernard, P. Živec and M. Gaberšček, Surf. Coat. Technol., 2011, 206, 1355–1361 CrossRef CAS PubMed.
- M. Tasbihi, U. Lavrenčič Štangar, A. Sever Škapin, A. Ristić, V. Kaučič and N. Novak Tušar, J. Photochem. Photobiol., A, 2010, 216, 167–178 CrossRef CAS PubMed.
- S. A. Larson, J. A. Widegren and J. L. Falconer, J. Catal., 1995, 157, 611–625 CrossRef CAS.
- Y. Ohko, A. Fujishima and K. Hashimoto, J. Phys. Chem. B, 1998, 102, 1724–1729 CrossRef CAS.
- M. Hirano, C. Nakahara, K. Ota, O. Tanaike and M. Inagaki, J. Solid State Chem., 2003, 170, 39–47 CrossRef CAS.
- Z. Liu, B. Guo, L. Hong and H. Jiang, J. Phys. Chem. Solids, 2005, 66, 161–167 CrossRef CAS PubMed.
- M. K. Akhtar, S. E. Pratsinis and S. V. R. Mastrangelo, J. Am. Ceram. Soc., 1992, 75, 3408–3416 CrossRef CAS PubMed.
- K. Okada, N. Yamamoto, Y. Kameshima, A. Yasumori and K. J. D. MacKenzie, J. Am. Ceram. Soc., 2001, 84, 1591–1596 CrossRef CAS PubMed.
- P. Zhang, Y. Yu, E. Wang, J. Wang, J. Yao and Y. Cao, ACS Appl. Mater. Interfaces, 2014, 6, 4622–4629 CAS.
- R. Rodriguez-Talavera, S. Vargas, R. Arroyo-Murillo, R. Montiel-Campos and E. Haro-Poniatowski, J. Mater. Res., 1997, 12, 439–443 CrossRef CAS.
- K. C. Song and S. E. Pratsinis, J. Am. Ceram. Soc., 2001, 84, 92–98 CrossRef CAS PubMed.
- R. A. Spurr and H. Myers, Anal. Chem., 1957, 29, 760–762 CrossRef CAS.
- D. M. Tobaldi, L. Gao, A. F. Gualtieri, A. Sever Škapin, A. Tucci and C. Giacobbe, J. Am. Ceram. Soc., 2012, 95, 1709–1716 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra14493g |
| This journal is © The Royal Society of Chemistry 2015 |