Metal-free ring expansion of indoles with nitroalkenes: a simple, modular approach to 3-substituted 2-quinolones†
Alexander V. Aksenov*a,
Alexander N. Smirnova,
Nicolai A. Aksenova,
Inna V. Aksenovaa,
Jonathon P. Mathenyb and
Michael Rubin*ab
aDepartment of Chemistry, North Caucasus Federal University, 1a Pushkin St., Stavropol 355009, Russian Federation. E-mail: alexaks@rambler.ru; Fax: +7 865 235 4033; Tel: +7 918 743 0255
bDepartment of Chemistry, University of Kansas, 1255 Wescoe Hall Dr., Lawrence, KS 66045-7582, USA. Fax: +1 785 864 5396; Tel: +1 785 864 5071
First published on 23rd December 2014
Abstract
3-Substituted 2-quinolones are obtained via a novel metal-free transannulation reaction of 2-nitroolefins with 2-substituted indoles in polyphosphoric acid. This acid-mediated cascade transformation operates via the ANRORC (Addition of Nucleophile, Ring Opening, and Ring Closure) mechanism and can be used in combination with the Fisher indole synthesis to offer a practical three-component hetero-annulation approach to 2-quinolones from arylhydrazines, 2-nitroalkenes, and acetophenone. An alternative entry to this chemistry employing the alkylation of electron-rich arenes and hetarenes with 1-(2-indolyl)-2-nitroalkene has also been demonstrated.
Introduction
3-Substituted 2-quinolones are attractive targets for medicinal chemistry1–3 and important tools for material science.4–6 (Fig. 1) shows a brief analysis of general approaches to 2-quinolones that include Vilsmeier–Haack (a),3d,7 Knorr (b),8 and Friedlander reactions (c).9 Along with transition metal-catalyzed versions of the above methods,10 and the recently emerged carbonylative cross-coupling reactions (d and f)11 and RCM (e),12 the cumulative prior art provides access to a wide variety of C3- and/or C4-substituted 2-quinolones. The two classical methods, Vilsmeier–Haack and Knorr, offer the advantage of employing readily available mono-substituted aromatic starting materials, whereas other described methods rely on availability of the more advanced ortho-disubstituted aromatic synthons.In contrast, approaches to 4-unsubstituted analogs are much less developed. Thus, general routes (a–c) provide poor results in reactions involving aldehydes or formamide derivatives (R3 = H). Du and Zhao disclosed an elegant metal-free approach to 3-aryl-2-quinolones (g) involving an iodine(III)-mediated cyclization with a 1,2-aryl shift, to produce 4-unsubstituted products.13 Recently, we communicated a new approach to a range of 3-aryl- and 3-alkylsubstituted 2-quinolones via a metal-free condensation reaction of readily available 2-substituted indoles with β-nitroalkenes proceeding via an ANRORC (Addition of Nucleophile, Ring Opening, and Ring Closure) pathway in polyphosphoric acid (PPA).14 We have also probed a one-pot preparation of 2-quinolones from arylhydrazines by merging this new methodology with Fisher indole synthesis. Herein we disclose a full account on this unusual transformation.
Results and discussion
Reaction of indoles with nitroalkenes
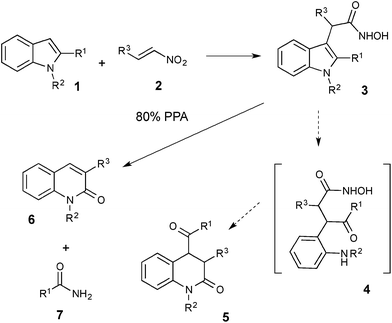
Our studies of the reactivity of nitro compounds with arenes in polyphosphoric acid15 have brought us to the serendipitous discovery of an unusual transannulation reaction of hydroxamic acid 3, formed upon electrophilic alkylation of indoles 1 with nitroolefins 2 (Scheme 1).14 Instead of the anticipated acid-assisted ring-opening of indole leading to 2(1H)-3,4-dihydroquinolinone 5, the test reaction between 2-phenylindole (1b) and β-nitrostyrene (2a) produced 3-phenyl-2-quinolone (6aa) lacking an acyl substituent at C4. Evidently, after a facile 5 → 6 ring expansion, the reaction took an unexpected turn: the R1 substituent of the indole together with the adjacent carbon atom (C2) was sacrificed to produce benzamide 7b as a byproduct, together with quinolone 6aa isolated in high yield (Table 1, entry 2).| 1 | R1 | R2 | 2 | R3 | 6 | Yielda, % | |
|---|---|---|---|---|---|---|---|
| a Isolated yields of purified 2-quinolones.b Hydroxamic acids were obtained as sole products under standard conditions. Their isolated yields are provided in parentheses. Indicated yields of 2-quinolones were obtained under forcing reaction conditions at 130 °C. | |||||||
| 1 | 1a | Me | H | 2a | Ph | 6aa | 90 |
| 2 | 1b | Ph | H | 2a | Ph | 6aa | 92 |
| 3 | 1a | Me | H | 2b | 4-MeOC6H4 | 6ab | 70 |
| 4 | 1b | Ph | H | 2b | 4-MeOC6H4 | 6ab | 74 |
| 5 | 1a | Me | H | 2c | 4-i-PrC6H4 | 6ac | 89 |
| 6 | 1a | Me | H | 2d | 3,4-Me2C6H3 | 6ad | 88 |
| 7 | 1a | Me | H | 2e | 3,4-(MeO)2C6H3 | 6ae | 78 |
| 8 | 1a | Me | H | 2f | 4-EtOC6H4 | 6af | 79 |
| 9 | 1a | Me | H | 2g | 2-FC6H4 | 6ag | 68 |
| 10 | 1a | Me | H | 2h | 3-FC6H4 | 6ah | 66 |
| 11 | 1a | Me | H | 2i | 4-FC6H4 | 6ai | 72 |
| 12 | 1a | Me | H | 2j | 3,4-Cl2C6H3 | 6aj | 88 |
| 13 | 1a | Me | H | 2k | 3-BrC6H4 | 6ak | 67 |
| 14 | 1a | Me | H | 2l | 2-C5H4N | 6al | 49 |
| 15 | 1b | Ph | H | 2l | 2-C5H4N | 6al | 46 |
| 16 | 1a | Me | H | 2m | 2-NO2C6H4 | 6am | 36 |
| 17 | 1b | Ph | H | 2m | 2-NO2C6H4 | 6am | 35 |
| 18 | 1a | Me | H | 2n | 2-BrC6H4 | 6an | NR |
| 19 | 1a | Me | H | 2o | 2,3-(MeO)2C6H3 | 6ao | NR |
| 20 | 1a | Me | H | 2p | n-Pr | 6ap | 63 |
| 21 | 1b | Ph | H | 2p | n-Pr | 6ap | 62 |
| 22 | 1a | Me | H | 2q | H | 6aq | 29 |
| 23 | 1c | Me | Me | 2q | H | 6cq | 27 |
| 24 | 1d | Ph | Me | 2q | H | 6cq | 11 |
| 25 | 1c | Me | Me | 2a | Ph | 6ca | 84b |
| 26 | 1c | Me | Me | 2i | 4-FC6H4 | 6ci | 74 (92)b |
| 27 | 1e | Me | n-Bu | 2a | Ph | 6ea | 24 (89)b |
| 28 | 1d | Ph | Me | 2a | Ph | 6ca | 3 (94)b |
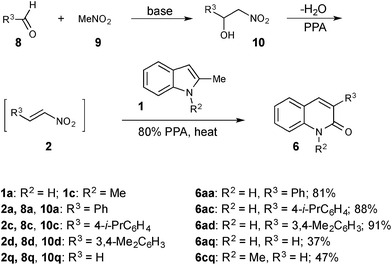
Subsequent optimization of the reaction conditions revealed that best results are produced upon heating a mixture of the 2-substituted indole (1) and the nitroalkene (2) in 80% PPA (a composition corresponding to diphosphoric acid, H4P2O7) at 80–85 °C for 30 min and then at 95–100 °C for additional 2.5–3 h. iso-Skatole (1a) has been identified as a more practical model substrate because it provides comparable yields of 2-quinolones (Table 1, compare entries 1–2, 3–4, 14–15, 16–17, 18–19, 20–21), but gives acetamide 7a as a byproduct, which is easily removable by routine aqueous workup. Systematic screening of various nitroolefins (Table 1) showed that electron-donating (entries 3–8) or weak electron-withdrawing groups (entries 9–13) on nitrostyrenes improve product yield. The reaction appeared to be very sensitive to sterics at the ortho-position of the nitrostyrenes: both 2-methyl- (1a) and 2-phenylindoles (1b) afforded low yield (entries 14–17) or no product at all (entries 18 and 19) with nitroalkenes bearing an ortho-substituent (2l–o). Aliphatic nitroolefin 2p reacted uneventfully with both model indoles (entries 20 and 21). Employment of nitroethene (2q) gave poor yields of products 6aq and 6cq lacking substituents at C3 (entries 22–24). This can be attributed to the reduced stability of 2q at elevated temperatures. Very different results were obtained in reactions of nitrostyrenes 2a and i with N-substituted indoles 1c–e, which upon exposure to standard reaction conditions exclusively produced the corresponding hydroxamic acids 3 (entries 26–28; yields are shown in parentheses). Attempts to force transannulation by increasing the reaction temperature to 130 °C resulted in good yields of products 6ca and 6ci starting from 1,2-dimethylindole (1c) (entries 25 and 26). However, high temperature reactions of indoles 1d and 1e, bearing a bulky substituent at nitrogen atom or at C2, resulted in substantial decomposition and poor yields of quinolones 6ca and 6ea (entries 27 and 28).
Three-component coupling involving Fisher indole synthesis
We envisioned a practical extension of this methodology in directly accessing the quinolines from easily available hydrazines by merging the Fisher indole synthesis16 with the above-described methodology in a one-pot transformation.17 The Fisher indole synthesis is known to proceed efficiently at elevated temperatures in orthophosphoric acid, so we anticipated 80% PPA (diphosphoric acid) to serve as a suitable medium for this reaction. Indeed, in our test experiment, a mixture of phenylhydrazine (11a) and acetophenone (12) heated in PPA at 100–110 °C quickly and cleanly produced desired indole 1b. In this case acetophenone was chosen for its high boiling point to alleviate material loss due to evaporation. Upon completion of the first step, nitroalkene 2a was added and subsequent heating of the reaction mixture afforded product 6aa in high yield (Scheme 2, Table 2, entry 1). Other nitroalkanes (Table 2, entries 2–13) as well as para-substituted hydrazines 11e–g (Table 2, entries 14–16) subjected to the one-pot transformation demonstrated efficiencies comparable to those obtained with pre-isolated indoles (Table 1).| 11 | R4 | 2 | R3 | 6 | Yielda, % | |
|---|---|---|---|---|---|---|
| a Isolated yields of purified products. | ||||||
| 1 | 11a | H | 2a | H | 6aa | 87 |
| 2 | 11a | H | 2b | 4-MeOC6H4 | 6ab | 68 |
| 3 | 11a | H | 2c | 4-i-PrC6H4 | 6ac | 85 |
| 4 | 11a | H | 2d | 3,4-Me2C6H3 | 6ad | 86 |
| 5 | 11a | H | 2e | 3,4-(MeO)2C6H3 | 6ae | 71 |
| 6 | 11a | H | 2f | 4-EtOC6H4 | 6af | 71 |
| 7 | 11a | H | 2g | 2-FC6H4 | 6ag | 62 |
| 8 | 11a | H | 2h | 3-FC6H4 | 6ah | 58 |
| 9 | 11a | H | 2i | 4-FC6H4 | 6ai | 66 |
| 10 | 11a | H | 2j | 3,4-Cl2C6H3 | 6aj | 82 |
| 11 | 11a | H | 2k | 3-BrC6H4 | 6ak | 60 |
| 12 | 11a | H | 2l | 2-C5H4N | 6al | 42 |
| 13 | 11a | H | 2m | 2-NO2C6H4 | 6am | 29 |
| 14 | 11e | Me | 2a | Ph | 6ha | 82 |
| 15 | 11f | MeO | 2a | Ph | 6fa | 79 |
| 16 | 11g | Cl | 2a | Ph | 6ga | 84 |
Reaction of indoles with 2-nitroethanols
The following modification of the standard protocol described above addresses some limitations of the Henry reaction,18 which was employed in this study to prepare starting nitroalkenes. The Henry reaction involves a base-assisted condensation of nitromethane 9 with corresponding aldehydes 8, and requires elimination of water in the last step, which proceeds smoothly for derivatives of aromatic aldehydes (R3 = Ar). Condensation of aliphatic aldehydes (R3 = Alk), unless carried out under much harsher conditions, often stops at the stage of alcohol 10 (Scheme 3). Having faced this problem, we reasoned that nitroalcohol 10 could potentially be employed in the described transformation as a surrogate of nitroolefin 2. Indeed, at elevated temperatures, PPA should force elimination of water producing in situ the required nitroalkene 2, which would subsequently react with indoles 1 (Scheme 3). Implementation of this idea afforded the corresponding 2-quinolones 6 in yields matching or exceeding those obtained via the original protocol (Scheme 3). This modification is particularly useful for the preparation of quinolones unsubstituted or alkyl-substituted at C3, for which the required 2-nitroethanols are much more readily available than the corresponding 2-nitroalkenes.Mechanistic rationale
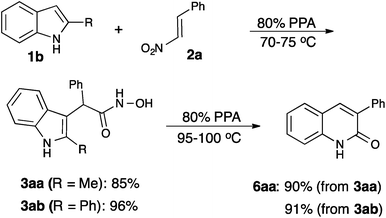
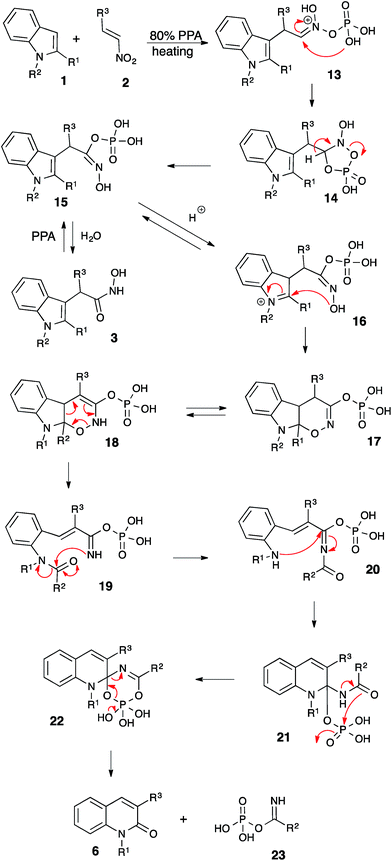
While detailed mechanistic study is underway in our laboratories, the ANRORC mechanism described below (Scheme 5) represents, in our opinion, the most plausible scenario out of several mechanistic hypotheses that could potentially account for the acquired body of empirical results, and is backed by literature precedence for the related elementary mechanistic steps.The reaction commences with the initial electrophilic attack by the nitroalkene at C3 of indole to produce alkylideneazinic acid 13. In the presence of PPA this aci-species undergoes rearrangement to hydroxamic acid anhydride 16, which upon hydrolysis provides hydroxamic acid 3.19 Indeed, acids 3aa (R1 = Me, R3 = Ph, R2 = H) and 3ab (R1 = R3 = Ph, R2 = H) were isolated as sole products after aqueous treatment of the mixtures when the reactions were carried out below 80 °C. When re-subjected to the standard reaction conditions, both 3aa and 3ab provided 2-quinolone 6aa in high yield (Scheme 4).
Subsequent steps involve intramolecular nucleophilic attack by the oxime moiety at the iminium functionality in 16 to afford tricyclic imine 17 (nucleophilic addition step of an ANRORC sequence), which in the presence of acid tautomerizes into enamine 18. The latter undergoes a retro-Diels–Alder reaction to produce anilide 19 (ring-opening step of an ANRORC sequence). Next, migration of the acyl group from aniline to the more nucleophilic imine nitrogen followed by the nucleophilic attack by the aniline at the acyliminium moiety in 20 (ring closure step of an ANRORC sequence)20 affords aminoquinoline species 21, which may cyclize into spiro-dioxaphosphazine 22. Finally, 2-quinolone 6 is produced after the extrusion of imide anhydride 23, which upon hydrolytic cleavage gives rise to the amide byproduct 7 (Scheme 5).
One of the possible alternative end-games involving formation of N-acylaminoquinoline 26 as an intermediate and its subsequent acid-assisted solvolysis into the final product has been ruled out. Indeed, if this mechanism were operating, compound 20 possessing R1 = H would quickly undergo aromatization into the thermodynamically more stable acylaminoquinoline 26 (Scheme 6). We demonstrated, however, that these compounds prepared by alternative methods do not show the expected reactivity under the featured reaction conditions.
3-Aryl-2-quinolones via arylation of nitroolefins
We have also envisioned an alternative route to 3-aryl-substituted 2-quinolones 6 via the electrophilic alkylation of arenes 28 with easily available21 3-indolyl nitroalkene 27. This mode would proceed via the key intermediate 13 (Scheme 5) and allow for easy diversification of an aromatic substituent R3 at the very last step of the synthesis. It would be particularly advantageous for the rapid assembly of 3-arylquinolone libraries22 as well as for efficient coupling of 2-quinolones with structurally advanced electron-rich aromatic fragments. To test this approach, nitroolefin 27 was treated with PPA in the presence of anisole under standard reaction conditions. We were pleased to find that this transformation proceeded smoothly to give the expected product 6ab (Scheme 7). Alkylation of other electron-rich arenes (o-xylene, veratrol, [1,3]-benzodioxole, phenetole, and tert-butylbenzene) afforded 3-arylsubstituted 2-quinolones 6ad, 6ae, 6ar, 6af, and 6as respectively, in good yields (Scheme 7). Application of this methodology for a single-step installation of a useful quinolone chromophore into a complex aromatic structure was also probed. Exposure of indole 27 and dibenzo-18-crown-6 ether to PPA under standard reaction conditions provided desired product 6at in 34% yield (Scheme 7), along with small amounts of two diastereomeric products resulted from alkylation of both benzene rings.Conclusion
We have developed a new, convenient and general approach to 3-substituted 2-quinolones via a metal-free cascade transformation starting from 2-substituted indoles and 2-nitroalkenes in polyphosphoric acid. The unique features of PPA that serves as a mild proton donor, a source for a good leaving group, a water scavenger, and a high-boiling solvent, make it an ideal medium for the described transformation. This uncatalyzed C–C bond forming reaction operates via an unusual ANRORC transannulation mechanism involving the extrusion of one carbon atom of an indole and incorporation of two new carbon atoms from a nitroolefin. This reaction was successfully combined with the Fisher indole synthesis, which led to the development of an efficient, sequential, three-component heteroannulation methodology for the construction of the 3-aryl-2-quinolone scaffold. This one-pot method offers a practical synthetic advantage over many known methods, which rely on 1,2-disubstituted aromatic precursors. Indeed, the featured methodology allows for the direct conversion of arylhydrazines into substituted 2-quinolones, which makes it very attractive for diversity oriented synthesis. An alternative entry to this transformation was explored, involving alkylation of electron-rich arenes with 2-indolyl nitroalkenes. This direct, non-catalytic C–H functionalization offers the possibility for an easy variability of the aromatic group at C3 of 2-quinolone.Experimental section
All reagents, solvents and catalysts were purchased from commercial sources and used without purification. All reactions were performed in oven-dried flasks open to the atmosphere and monitored by thin layer chromatography. Flash column chromatography was performed on silica gel (32–63 μm, 60 Å pore size). Nitroalkenes 2a,b,e,g,h,j,k, m,n,o were obtained from commercial sources, compounds 2c,d,f,i,l (ref. 23) and 2p,q (ref. 24) were synthesized employing published procedures. Commercial 2-nitroethanole (10q) was used. 1-Aryl-2-nitroethanoles 10a,c,d were synthesized using standard literature procedure.25Reactions of 2-substituted indoles with 2-nitroalkenes in PPA
Reaction of 3-nitrovinylindoles with arenes
A mixture of 3-nitrovinylindole 27 (1 mmol), selected arene (1.2 mmol) and PPA (2–3 g) was heated at 80–85 °C for 30 min. The consumption of starting materials was monitored by TLC. After indole reacted completely, the temperature was increased to 95–100 °C and the reaction mixture was heated for 1 h, then cooled to room temperature, poured into water (50 mL) and neutralized with aqueous ammonia. The organic portion was extracted twice with chloroform and filtered through silica gel. Concentration of the filtrate in vacuum followed recrystallization of the residue afforded the corresponding 2-quinolones.Experiments on isolation of intermediate hydroxamic acid and its further conversion into 2-quinolone
A mixture of indole 1a,d,c,e (1.0 mmol), β-nitrostyrene 2a,i (1.2 mmol) and PPA (4 g) was stirred at 70–75 °C for 30 min. The reaction mixture was cooled down to room temperature, poured into water (50 mL), and neutralized by aqueous ammonia to pH ∼8. The formed precipitate was filtered off and recrystallized.Hydroxamic acids 3aa,ab,db,ci,ea obtained as described above (1 mmol) were stirred in PPA at 95–100 °C for 3 h. The mixtures were cooled down, poured into water (50 mL), and neutralized with aqueous ammonia to pH ∼8. The aqueous portions were extracted with chloroform (2 × 50 mL) and combined organic phases were filtered through a short pad of silica gel (32–63 μm, 60 Å pore size). The filtrates were concentrated in vacuum to afford samples of quinolones 6aa,cq,ci,ea identical to the material described above.
Acknowledgements
This work was supported by the Russian Science Foundation (grant #14-23-00068).Notes and references
- For recent reviews, see: (a) C. B. M. Poulie and L. Bunch, ChemMedChem, 2013, 8, 205–215 CrossRef CAS PubMed; (b) S. Heeb, M. P. Fletcher, S. R. Chhabra, S. P. Diggle, P. Williams and M. Camara, FEMS Microbiol. Rev., 2011, 35, 247–274 CrossRef CAS PubMed.
- For recent examples, see: (a) D. A. Sabbah, N. A. Simms, W. Wang, Y. Dong, E. L. Ezell, M. G. Brattain, J. L. Vennerstrom and H. A. Zhong, Bioorg. Med. Chem., 2012, 20, 7175–7183 CrossRef CAS PubMed; (b) N. Kumar, V. P. Raj, B. S. Jayshree, S. S. Kar, A. Anandam, S. Thomas, P. Jain, A. Rai and C. M. Rao, Chem. Biol. Drug Des., 2012, 80, 291–299 CrossRef CAS PubMed; (c) A. A. Al-Amiery, R. I. H. Al-Bayati, K. Y. Saour and M. F. Radi, Res. Chem. Intermed., 2012, 38, 559–569 CrossRef CAS PubMed; (d) D. Beattie, D. Beer, M. E. Bradley, I. Bruce, S. J. Charlton, B. M. Cuenoud, R. A. Fairhurst, D. Farr, J. R. Fozard, D. Janus, E. M. Rosethorne, D. A. Sandham, D. A. Sykes, A. Trifilieff, K. L. Turner and E. Wissler, Bioorg. Med. Chem. Lett., 2012, 22, 6280–6285 CrossRef CAS PubMed.
- (a) A. Doléans-Jordheim, J. B. Veron, O. Fendrich, E. Bergeron, A. Montagut-Romans, Y. S. Wong, B. Furdui, J. Freney, C. Dumontet and A. Boumendjel, ChemMedChem, 2013, 8, 652–657 CrossRef PubMed; (b) S. E. Wolkenberg, Z. Zhao, C. Thut, J. W. Maxwell, T. P. McDonald, F. Kinose, M. Reilly, C. W. Lindsley and G. D. Hartman, J. Med. Chem., 2011, 54, 2351–2358 CrossRef CAS PubMed; (c) P. V. Chaturvedula, S. E. Mercer, S. S. Pin, G. Thalody, C. Xu, C. M. Conway, D. Keavy, L. Signor, G. H. Cantor, N. Mathias, P. Moench, R. Denton, R. Macci, R. Schartman, V. Whiterock, C. Davis, J. E. Macor and G. M. Dubowchik, Bioorg. Med. Chem. Lett., 2013, 23, 3157–3161 CrossRef CAS PubMed; (d) B. Joseph, F. Darro, A. Behard, B. Lesur, F. Collignon, C. Decaestecker, A. Frydman, G. Guillaumet and R. J. Kiss, J. Med. Chem., 2002, 45, 2543–2555 CrossRef CAS PubMed.
- See for example: W. M. F. Fabian, K. S. Niederreiter, G. Uray and W. Stadlbauer, J. Mol. Struct., 1999, 477, 209–220 CrossRef CAS.
- See for example: M. S. Tremblay, M. Halim and D. Sames, J. Am. Chem. Soc., 2007, 129, 7570–7577 CrossRef CAS PubMed.
- L. Clima and W. Bannwarth, Helv. Chim. Acta, 2008, 91, 165–175 CrossRef CAS.
- See, for example: (a) Y. Zhang, Y. Fang, H. Liang, H. Wang, K. Hu, X. Liu, X. Yi and Y. Peng, Bioorg. Med. Chem. Lett., 2013, 23, 107–111 CrossRef CAS PubMed; (b) M. A. Alonso, M. M. Blanco, C. Avendano and J. C. Menendez, Heterocycles, 1993, 36, 2315–2325 CrossRef CAS.
- See, for example: (a) X. Liu, X. Xin, D. Xiang, R. Zhang, S. Kumar, F. Zhou and D. Dong, Org. Biomol. Chem., 2012, 10, 5643–5646 RSC; (b) G. Uray, K. S. Niederreiter, F. Belaj and W. M. F. Fabian, Helv. Chim. Acta, 1999, 82, 1408–1417 CrossRef CAS.
- See, for example: (a) S.-Y. Han, J. W. Choi, J. Yang, C. H. Chae, J. Lee, H. Jung, K. Lee, J. D. Ha, H. R. Kim and S. Y. Cho, Bioorg. Med. Chem., 2012, 22, 2837–2844 CrossRef CAS PubMed; (b) K. K. Park and J. Y. Jung, Heterocycles, 2005, 65, 2095–2105 CrossRef CAS PubMed.
- (a) P. J. Manley and M. T. Bilodeau, Org. Lett., 2004, 6, 2433–2435 CrossRef CAS PubMed; (b) L. Fu, X. Huang, D. Wang, P. Zhao and K. Ding, Synthesis, 2011, 1547–1574 CAS.
- (a) J.-R. Chen, J. Liao and W.-J. Xiao, Can. J. Chem., 2010, 88, 331–337 CrossRef CAS PubMed; (b) D. V. Kadnikov and R. C. Larock, J. Organomet. Chem., 2003, 687, 425–435 CrossRef CAS; (c) A. C. Tadd, A. Matsuno, M. R. Fielding and M. C. Willis, Org. Lett., 2009, 11, 583–586 CrossRef CAS PubMed.
- J. Minville, J. Poulin, C. Dufresne and C. F. Sturino, Tetrahedron Lett., 2008, 49, 3677–3681 CrossRef CAS PubMed.
- L. Liu, H. Lu, H. Wang, C. Yang, X. Zhang, D. Zhang-Negrerie, Y. Du and K. Zhao, Org. Lett., 2013, 15, 2906–2909 CrossRef CAS PubMed.
- A. V. Aksenov, A. N. Smirnov, N. A. Aksenov, I. V. Aksenova, L. V. Frolova, A. Kornienko, I. V. Magedov and M. Rubin, Chem. Commun., 2013, 49, 9305–9307 RSC.
- For review, see: (a) A. N. Smirnov, N. A. Aksenov, I. V. Malikova and A. V. Aksenov, Chem. Heterocycl. Compd., 2014, 50, 594–618 CrossRef CAS PubMed , For recent studies, see:; (b) S. V. Shcherbakov, D. A. Lobach, M. Rubin and A. V. Aksenov, Chem. Heterocycl. Compd., 2014, 50, 757–760 CrossRef CAS PubMed; (c) A. V. Aksenov, N. A. Aksenov, A. E. Tsys', V. I. Goncharov and S. N. Ovcharov, Russ. Chem. Bull., 2013, 62, 1127–1128 CrossRef CAS PubMed; (d) N. A. Aksenov, A. V. Aksenov, I. V. Aksenova and Y. I. Smushkevich, Chem. Heterocycl. Compd., 2013, 49, 645–647 CrossRef CAS PubMed; (e) A. V. Aksenov, N. A. Aksenov, O. N. Nadein and I. V. Aksenova, Synth. Commun., 2012, 42, 541–547 CrossRef CAS; (f) A. V. Aksenov, N. A. Aksenov, O. N. Nadein and I. V. Aksenova, Synlett, 2010, 2628–2630 CrossRef CAS PubMed.
- For reviews, see: (a) B. Robinson, Chem. Rev., 1969, 69, 227–250 CrossRef CAS; (b) B. Robinson, Chem. Rev., 1963, 63, 373–401 CrossRef; (c) G. W. Gribble, J. Chem. Soc., Perkin Trans. 1, 2000, 1045–1075 RSC; (d) G. R. Humphrey and J. T. Kuethe, Chem. Rev., 2006, 106, 2875–2911 CrossRef CAS PubMed.
- An alternative mode of cascade combining the Fisher indolization with the ANRORC reaction featured herein, was recently communicated, see: A. V. Aksenov, A. N. Smirnov, N. A. Aksenov, I. V. Aksenova, A. S. Bijieva and M. Rubin, Org. Biomol. Chem., 2014, 12, 9786–9788 CAS.
- (a) C. Palomo, M. Oiarbide and A. Laso, Eur. J. Org. Chem., 2007, 2561–2574 CrossRef CAS; (b) F. A. Luzzio, Tetrahedron, 2001, 57, 915–945 CrossRef CAS.
- For alkylation of arenes with 2-nitrostyrenes to afford hydroxamic acids, see: A. D. Grebenyuk and A. K. Tashmukhamedova, Chem. Heterocycl. Compd., 2006, 42, 732–734 CrossRef CAS.
- Apparently, E → Z isomerization of an olefin moiety should accompany this cyclization. We believe this process can occur easily due to reversible conjugate addition of any adventurous nucleophilic species across this double bond.
- L. Canoira, J. Gonzalo Rodriguez, J. B. Subirats, J. A. Escario, I. Jimenez and A. R. Martinez-Fernandez, Eur. J. Med. Chem., 1989, 24, 39–42 CrossRef CAS.
- For screening of 3-aryl-2-quinolone library to evaluate their anti-tumor activity, see: M. Mehta, J. W. Keisinger, X. P. Zhang, M. L. Lerner, D. J. Brackett, R. W. Brueggemeier, P.-K. Li and J. T. Pento, Anticancer Res., 2010, 30, 4883–4889 CAS.
- (a) D. E. Worral, J. Am. Chem. Soc., 1938, 60, 2841 CrossRef; (b) K. W. Rosenmund, Chem. Ber., 1909, 42, 4778 CrossRef; (c) A. B. Eltsov, Russ. J. Gen. Chem., 1962, 32, 1525 CAS.
- (a) E. Schmidt and G. Rutz, Chem. Ber., 1928, 61, 2142–2148 CrossRef; (b) G. D. Buckley and C. W. Scaife, J. Chem. Soc., 1947, 1471–1472 RSC.
- O. Snow, Psychoactive Synthesis Series, Amphetamine Syntheses: Overview & Reference Guide for Professionals, Thoth Press, USA, 1998, vol. 1, p. 82 Search PubMed.
- K. Hino, Y. Nagai and H. Uno, Chem. Pharm. Bull., 1987, 35, 2819–2824 CrossRef CAS.
- D. H. Hey and J. M. Williams, J. Chem. Soc., 1950, 1678–1683 RSC.
- P. M. Fresneda, P. Molina and S. Delgado, Tetrahedron, 2001, 57, 6197–6202 CrossRef CAS.
- K. K. Park and J. Y. Jung, Heterocycles, 2005, 65, 2095–2105 CrossRef CAS PubMed.
- Y.-J. Cherng, Tetrahedron, 2002, 58, 1125–1129 CrossRef CAS.
- C. E. Kaslow and B. Buncher, J. Org. Chem., 1958, 23, 271–276 CrossRef CAS.
- H. Ahlbrecht and C. Vonderheid, Chem. Ber., 1975, 108, 2300–2311 CrossRef CAS.
- T. Eicher and V. Schneider, Synthesis, 1989, 372–378 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: 1H, 13C NMR and HRMS spectral charts for all new compounds. See DOI: 10.1039/c4ra14406f |
| This journal is © The Royal Society of Chemistry 2015 |