3D electron microscopy investigations of human dentin at the micro/nano-scale using focused ion beam based nanostructuring
Meltem Sezen*a and
Sina Sadighikiaab
aSabanci University Nanotechnology Research and Application Center (SUNUM), Orhanli-Tuzla, 34956, Istanbul, Turkey. E-mail: meltemsezen@sabanciuniv.edu
bFaculty of Natural Sciences and Engineering, Sabanci University, Orhanli, Tuzla, 34956, Istanbul, Turkey
First published on 19th December 2014
Abstract
In this study, high resolution electron microscopy techniques, such as Focused Ion Beam (FIB), Scanning Electron Microscopy (SEM) and High Resolution Transmission Electron Microscopy (HRTEM) revealed micro and nano features within human dentin with high definition and accuracy. The samples were prepared using FIB based advanced nanostructuring techniques in a dual-beam instrument. The related secondary electron (SE) image tomographs were acquired by means of stacking the images from FIB slice-series for monitoring micro-sized dentinal tubules, whereas FIB-structured pin-like samples were investigated at the TEM to observe the collagen fibrils at the nanoscale. The complimentary analysis helped to reveal the microstructure and morphology of human dentin in three dimensions in detail.
Introduction
All materials and structures that have features in three dimensions need to be investigated in detail using 3D characterization techniques. When biomaterials are considered, in particular, hard tissues; e.g. teeth, bones, joints are of high interest in this class, while such materials have both micro- and nanometer sized channels, pores and features within the structure. X-ray tomography applications are often insufficient for deep analysis due to the fact that, X-ray tomographs are able to provide data with an imaging resolution down to a few hundreds of nanometers. When small features are to be observed, 3D electron microscopy techniques are preferred for their high-resolution capabilities and high precise and more reliable processing abilities as far as imaging, micro/nano-structuring and data evaluation stages are concerned.Dentin is a hydrated hard tissue that comprises the majority of human teeth by both weight and volume.1 The tissue serves as an elastic foundation for the hard, outermost enamel, and as a protective enclosure for the central pulp. Dentin consists of microscopic channels, called dentinal tubules, which radiate outward through the dentin from the pulp to the exterior cementum or enamel border. These tubules contain fluid and cellular structures.2 As a result, dentin has a degree of permeability, which can increase the sensation of pain and the rate of tooth decay. Dentin is traversed by a network of tubules that are oriented radially outward from the central pulp towards the dentin–enamel junction.1
On the other hand, type I collagen forms a fibrous three-dimensional network structure which build up the dentin matrix. Compared to bone, the collagen matrix in dentin is more interwoven with numerous crossing of fibrils.3 The layers of a human tooth are demonstrated in Fig. 1.
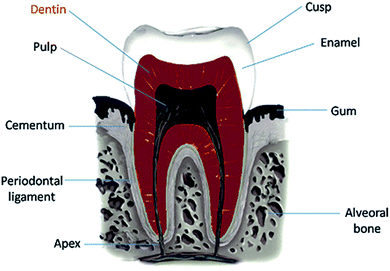 | ||
| Fig. 1 The scheme of a human tooth showing the individual layers. The dentin layer (marked in red) containing tubules was of interest in this study. | ||
As the demand for providing information at nano and sub-nano scale especially in the field of “life sciences” increases, the scientists are in search of employing cutting-edge analysis technologies and developing novel methodologies over advanced characterization tools. Focused Ion Beams (FIB) is one of the outstanding technologies as far as structuring, modification and prototyping from micro- down to nanometer scales are concerned. Dual beam platforms, consisting of a HR-Scanning Electron Microscope (SEM) and a Focused Ion Beam (FIB) column, additionally equipped with gas injection systems and micromanipulators serve as multi-functional tools for nano-structuring and nano-tomography; as well as specimen preparation for Transmission Electron Microscopy (TEM) can be carried out with ultra-high precision. Ion milling techniques are highly important as far fragile hard materials are considered due to the fact that cross-sectioning and TEM specimen preparation are problematic for these materials via conventional mechanical sectioning or thinning techniques, such as ultramicrotomy and dimpling.
In this study, firstly, FIB-tomography applications in dual-beam platforms were employed for revealing the morphology of tooth tissues both at nano and micro scale for a better understanding of the distribution of tubules throughout the dentin layer. In order to achieve 3D information, serial slicing by gallium ions was performed and electron image from each cross-section was acquired. This was followed by stacking 2D SE-images into 3D volume via reconstruction software in order to form a micro-tomograph.4 In the other hand, the use of pin-like sample allowed for avoiding projection problems during TEM imaging and tomography tilt series.
In addition, previously, it has been reported in the literature that FIB/SEM dual-beam platforms have been extensively used on dental structures, such as dentin or enamel, for either specimen preparation for TEM5–8 or 3D micro structural analysis using slice & view methods.9 However there has been no complimentary study yet, that combines the investigation on the micron-sized features of dentin (e.g. tubules) with the nano-features (e.g. collagen fibrils) using both the nanostructuring facilities of dual-beam platforms and the TEM analysis on the FIB-processed 3D specimens.
Experimental
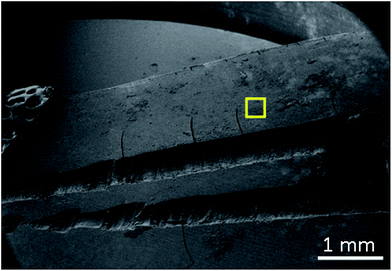
Before electron microscopy investigations, a human tooth was mechanically cross-sectioned with a die for a better view of layers within the structure. As shown in Fig. 2, the tooth was channel treated and had fillings in the channels nearby dentin layer. In order to do 3D micro structural investigation by means of slice & view based tomography, the region of interest was chosen as the area marked by yellow square in the figure. Prior to dual-beam applications performed on the sample, in order to avoid charging effects that are caused by electron beams, the sample was gold-plated using sputter coater. The thickness of gold coating was approximately 10 nm.For the FIB slice & view series and the following 3D construction a region of approximately 18 × 17 × 16 μm was chosen. Within this region, via ion milling, 332 slides were cut with a 50 nm step, using 1 nA ion current at 30 keV ion energy. The secondary electron (SE) images of the slice & view series was acquired at 2500× magnification simultaneously from the cross-sectioned regions. This work has been carried out at a dual-beam platform from JEOL Company: JIB 4601F MultiBeam System having a FEG SEM column and a gallium-ion column, equipped with gas injection systems and a micromanipulator. The related SE images were then processed using Stack N Viz software in order to achieve a 3D reconstructed tomograph in 3D.
In order to track the nano-sized features within the dentin structure, such as collagen fibrils, the sample needed to be investigated in TEM. Due to the difficulties of site-specific sample preparation from hard materials by other methods, the TEM specimen preparation process was also carried out by the help of a dual-beam platform. The specimen preparation process in the dual-beam platforms was performed using in situ lift-out technique by the help of a micromanipulator. In order to prevent gallium implantation, initially two protective platinum layers were deposited on top of the region of interest before trenching with annular patterns. The free sections were then mounted on a TEM grid and thinned further by ion milling until thin samples (around 100 nm) were formed. In the final stage, TEM samples were polished, using rather low ion currents for the removal of gallium which might have been implanted on the specimen surface during the milling process.
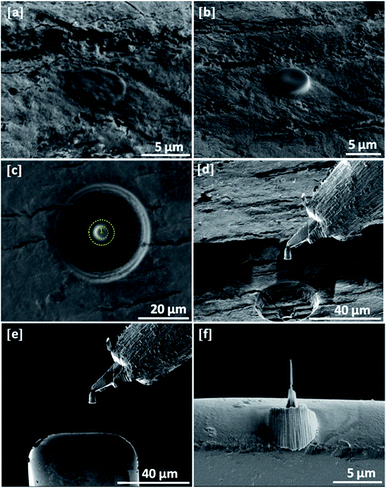
As a result, electron transparent and uniform pin-like specimens were achieved. The uniformity of pin-form geometry provides detailed morphological and information in three-dimensions (3D) while it is possible to acquire 3D data for revealing the collagen fibrils within the dentinal tissues. For this work an FEI NanoLab Nova 600 DualBeam platform was used. The SE images corresponding to the stages of pin-like TEM specimen preparation are given by Fig. 3.
The preparation steps can be summarized as following: (a) the deposition of protective electron beam assisted platinum layer (with a diameter of 5 μm) onto the region of interest; (b) the deposition of protective ion beam assisted platinum layer (with a diameter of 5 μm) onto the top of electron beam assisted deposit; (c) ion milling of pillar shape sections using intermediate ion beam currents (e.g. 0.1–1 nA) within annular patterns (the yellow circle corresponds to the annular pattern); (d) the lift-out of the section from the dentin structure using a micromanipulator; (e) mounting the pillar to the TEM grid using micromanipulator and platinum deposition; (f) final thinning of the sample using low ion currents (e.g. 10–100 pA).
Results and discussions
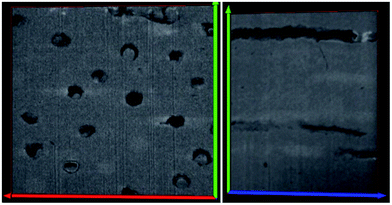
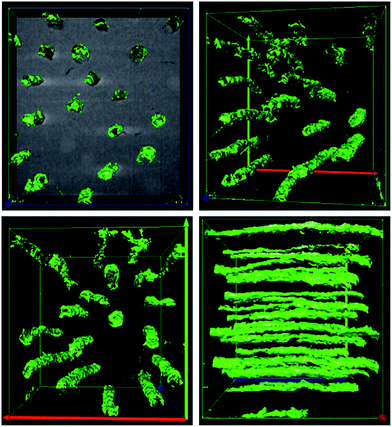
This work presents the micro and nano features within human dentin with high definition and accuracy by means of, high resolution electron microscopy techniques. The major advantage over other imaging methods is the ability to track the features within the dentin 3D space. The results provide the following information: (i) the tubule distribution, alignment and orientation within the structure which is available by slice and view technique in dual beam platform. The different images of dentinal tubules distribution are shown in Fig. 4 and 5. The mean number of tubules inside a 342 μm2 surface for 332 slides is about 13. This gives a number for the tubular density of approximately 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 consistent, with previous 2D studies.10 However 3 dimensional observation makes it much easier to examine the tubule number densities, (ii) in next step, pin-like thin samples were investigated in a FEI TF30 HR-TEM system and BF images were acquired at 300 keV electron energy.
000 consistent, with previous 2D studies.10 However 3 dimensional observation makes it much easier to examine the tubule number densities, (ii) in next step, pin-like thin samples were investigated in a FEI TF30 HR-TEM system and BF images were acquired at 300 keV electron energy.
The TEM micrographs revealed the 3D network of collagen fibrils within the dentin structure. The corresponding micrographs are given in Fig. 6. Transparent and normal dentin inter-tubular mineral crystallites appeared needle-like in morphology when observed on edge, similar to previous observations of dentin by other groups.11,12 Thus, the TEM analysis revealed the nano-features e.g. collagen fibrils that are present in the dentin structure, while the FIB work was useful for tracking the micro-sized features such as dentinal tubules. It should be noted that, FIB was the main instrument used for the micro/nano-machining of both structures: tomography sections on the material and the pin-like TEM specimen.
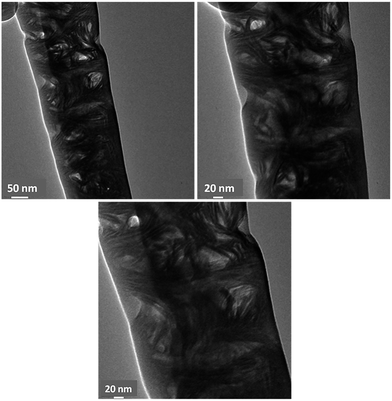 | ||
| Fig. 6 The Bright Field (BF) TEM images showing the 3D distribution of collagen fibrils within the human dentin. The micrographs show the nano features within the dentin structure. | ||
Conclusions
In this work, complimentary electron microscopy analysis including Focused Ion Beam (FIB), Scanning Electron Microscopy (SEM) and high resolution Transmission Electron Microscopy (TEM) were used for observing micro and nano-details within human dentin. Dual-beam instruments allowed for preparing pin-like uniform and representative 3D TEM specimens using ion-milling and deposition based advanced nanostructuring techniques. This was followed by acquiring related secondary electron (SE) image tomographs of stacking the images from FIB slice-series for monitoring micro-sized dentinal tubules, whereas FIB-structured pin-like samples were investigated at the TEM for observing the nanometer sized collagen fibrils that form dentinal tissues. Consequently, this study enabled for revealing the microstructure and morphology of human dentin in three dimensions for both its micro and nano constituents.Acknowledgements
The authors would like to thank Burak Kıtıki at Marmara University, Faculty, of Dentistry, Istanbul for providing tooth samples; Mustafa Güler at Bilkent University, UNAM, Ankara for his support for TEM investigations and JEOL Company for providing 3D-FIB-Tomography constructions. Also, support by TUBITAK 112M195 Project and COST MP1103 Action are gratefully acknowledged.Notes and references
- D. D. Arola and R. K. Reprogel, Biomaterials, 2006, 27(9), 2131–2140 CrossRef CAS PubMed.
- M. H. Ross, I. K. Gordon and W. Pawlina, Histology: a text and atlas, 4th edn, 2003, p. 450 Search PubMed.
- S. Habelitz, M. Balooch, S. J. Marshall, G. Balooch and G. W. Marshall, J. Struct. Biol., 2002, 138, 227–236 CrossRef CAS.
- T. Uusimäki, G. Margaris, K. Trohidou, P. Granitzer, K. Rumpf, M. Sezen and G. Kothleitner, Nanoscale, 2013, 5, 11944–11953 RSC.
- A. E. Porter, R. K. Nalla, A. Minora, J. R. Jinschek, C. Kisielowskia, V. Radmilovica, J. H. Kinney, A. P. Tomsia and R. O. Ritchieb, Biomaterials, 2005, 26, 7650–7660 CrossRef CAS PubMed.
- S. Duarte Jr, A. Avishai and A. Sadan, Microsc. Microanal., 2009, 15, 368–369 CrossRef.
- R. K. Nallaa, A. E. Porter, C. Daraioc, A. M. Minorb, V. Radmilovic, E. A. Stachb, A. P. Tomsiaa and R. O. Ritchie, Micron, 2005, 36, 672–680 CrossRef PubMed.
- K. Hoshi, S. Ejiri, W. Probst, V. Seybold, T. Kamino, T. Yaghuchi, N. Yamahira and H. Ozawa, Microscopy, 2001, 201, 44–49 CrossRef CAS.
- J. S. Earl, R. K. Leary, J. S. Perrin, R. Brydson, J. P. Harrington, K. Markowitz and S. J. Milne, Microscopy, 2010, 240, 1–5 CrossRef CAS PubMed.
- I. A. Mjor and I. Nordahl, Arch. Oral Biol., 1996, 41(5), 401–412 CrossRef CAS.
- J. C. Voegel and R. M. Frank, J. Biol. Buccale, 1977, 5, 181–194 CAS.
- V. Ziv and S. Weiner, Connect. Tissue Res., 1994, 30, 165–175 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |