One-pot conversion of carbohydrates into gamma-valerolactone catalyzed by highly cross-linked ionic liquid polymer and Co/TiO2
Abstract
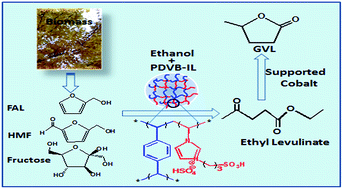
The acid catalytic conversion of carbohydrates into levulinate esters followed by metal catalytic hydrogenation is an important approach to obtain gamma-valerolactone (GVL) from biomass. In this work, we prepared the highly cross-linked polymer of divinylbenzene with acid ionic liquid (PDVB-IL) and supported Co on TiO2 (Co/TiO2), which were characterized by Fourier transform infrared (FTIR) spectroscopy, scanning electron microscopy (SEM), transmission electron microscopy (TEM), and elemental analysis. It was demonstrated that the PDVB-IL polymer could efficiently catalyze the esterification reaction of furfuryl alcohol (FAL), 5-hydroxymethylfurfural (HMF), and fructose with ethanol to produce the intermediate ethyl levulinate (EL), and the EL in the reaction mixture was directly hydrogenated to GVL over the Co/TiO2 without requiring purification and with high yields.

 Please wait while we load your content...
Please wait while we load your content...