Sol–gel hydrothermal synthesis of microstructured CaO-based adsorbents for CO2 capture†
Abstract
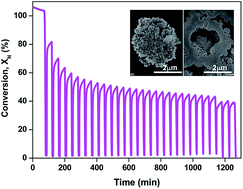
In this study, microstructured CaO-based adsorbents were synthesized by a sol–gel hydrothermal method using calcium nitrate tetrahydrate, citric acid and sodium hydroxide as precursors. Experiments with different NaOH concentrations (2, 6 and 10 M) were carried out to investigate the effects on the morphologies and CO2 adsorption activities of the synthesized adsorbents. X-ray diffraction (XRD) and field-emission scanning electron microscopy (FESEM) results showed that different NaOH concentrations resulted in different crystal phases and morphologies. A novel three-dimensional (3D) hierarchical calcite (CaCO3) hollow microspherical adsorbent composed of one-dimensional (1D) spike-shaped nanorods was obtained with 2 M NaOH. XRD analyses confirmed that the hierarchical CaCO3 hollow microspheres were characteristic of the calcite phase. The FESEM image revealed that the microspheres were composed of 1D spike-shaped nanorods with an average length of 500 nm. The cross-sectional FESEM image showed that the microspheres had hollow structures with an average inner cavity of 2 μm and a shell thickness of approximately 0.5 μm. The CO2 adsorption performance of the synthesized adsorbents was investigated using thermogravimetry-differential thermal analysis (TG-DTA) apparatus. The results indicated that the novel hierarchical calcite (CaCO3) hollow microspherical adsorbent composed of one-dimensional (1D) spike-shaped nanorods possessed higher carbonation conversion of 45% after 15 cycles, which was about 22% higher than that of other adsorbents synthesized with 6 and 10 M NaOH concentration and limestone. This property could be attributed to the 3D hierarchical hollow microsphere structure, 1D spike-shaped nanorod structure, trimodal pore size distribution and large BET surface area (44.85 m2 g−1) of the novel adsorbent.


 Please wait while we load your content...
Please wait while we load your content...