Highly heat resistant and thermo-oxidatively stable borosilane alkynyl hybrid polymers
Abstract
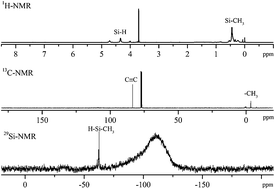
A type of boron–silicon containing hybrid polymer with C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C units (HBS) was prepared, and the effects of three different substituents on the properties of the polymers were studied. The polymers were synthesized with ethynylmagnesium bromide, dichlorosilane and boron fluoride etherate by using a Grignard reagent method. The structures of HBS were characterized by Fourier-transform infrared spectra, proton-NMR (1H-NMR), 13C-NMR, 29Si-NMR, and gel permeation chromatography. Thermal and oxidative stabilities were studied using differential scanning calorimetry and thermogravimetry analysis, and the crosslinking reaction mechanisms of the HBS are discussed. All the polymers exhibit excellent thermal and oxidation resistance, particularly, HBS-1 with Si–H bonds which was highly heat resistant and showed good thermo-oxidative stability. The temperatures of 5% weight loss (Td5) were 624 °C and 607 °C in nitrogen and air, respectively, and the residues at 1000 °C were 86.8% and 77.5%, respectively. The thermal and oxidative stabilities of the polymers were attributed to the synergistic effect of boron and silicon elements and the cross linking during hydrosilylation and Diels–Alder reactions.
C units (HBS) was prepared, and the effects of three different substituents on the properties of the polymers were studied. The polymers were synthesized with ethynylmagnesium bromide, dichlorosilane and boron fluoride etherate by using a Grignard reagent method. The structures of HBS were characterized by Fourier-transform infrared spectra, proton-NMR (1H-NMR), 13C-NMR, 29Si-NMR, and gel permeation chromatography. Thermal and oxidative stabilities were studied using differential scanning calorimetry and thermogravimetry analysis, and the crosslinking reaction mechanisms of the HBS are discussed. All the polymers exhibit excellent thermal and oxidation resistance, particularly, HBS-1 with Si–H bonds which was highly heat resistant and showed good thermo-oxidative stability. The temperatures of 5% weight loss (Td5) were 624 °C and 607 °C in nitrogen and air, respectively, and the residues at 1000 °C were 86.8% and 77.5%, respectively. The thermal and oxidative stabilities of the polymers were attributed to the synergistic effect of boron and silicon elements and the cross linking during hydrosilylation and Diels–Alder reactions.

 Please wait while we load your content...
Please wait while we load your content...