Chemical composition and potential bioactivity of strawberry pomace†
Vesna Tumbas Šaponjac*a,
Amadeo Gironés-Vilaplanab,
Sonja Djilasa,
Pedro Menab,
Gordana Ćetkovića,
Diego A. Morenob,
Jasna Čanadanović-Bruneta,
Jelena Vulića,
Slađana Stajčića and
Milica Vinčića
aFaculty of Technology, University of Novi Sad, Bulevar cara Lazara 1, 21000 Novi Sad, Serbia. E-mail: vesnat@uns.ac.rs; Fax: +381 21 450 413; Tel: +381 21 485 3763
bPhytochemistry Lab., Department of Food Science and Technology, CEBAS-CSIC, Campus de Espinardo-25, E-30100, Espinardo, Murcia, Spain
First published on 11th December 2014
Abstract
This study has been designed to screen phenolic compounds of strawberry pomace obtained from two strawberry cultivars, ‘Clery’ (SC) and ‘Marmolada’ (SM), and their biological activity (O2˙−, ˙OH, DPPH˙ and ABTS+˙ antioxidant capacity, α-glucosidase inhibition and reducing power). The SM pomace contained significantly higher (p < 0.05) amounts of total flavonoids and anthocyanins, individual phenolic acids and flavonoids, among them protocatechuic acid, catechin and pelargonidin-3-glucoside (1838.31 and 1646.68 per 100 g DW, respectively) being the most abundant. The SM pomace showed higher DPPH˙ and ABTS+˙ antioxidant capacities and reducing power, as well as higher activity towards O2˙−  , ˙OH (EC50˙OH = 2.77 mg mL−1) and α-glucosidase inhibitory potential (ECα-GIP50 = 1.16 mg mL−1). Our results support the use of strawberry pomace as a rich source of phytochemicals for further utilization in the food industry as supplements or ingredients for food fortification.
, ˙OH (EC50˙OH = 2.77 mg mL−1) and α-glucosidase inhibitory potential (ECα-GIP50 = 1.16 mg mL−1). Our results support the use of strawberry pomace as a rich source of phytochemicals for further utilization in the food industry as supplements or ingredients for food fortification.
1. Introduction
The importance of a diet rich in fruits and vegetables is widely cited in the literature as it is connected with a reduced risk of oxidative stress and chronic non-communicable diseases and cardiovascular conditions.1 The nutritional and health benefits of plant derived foods may be partially due to their high content of certain phenolic compounds.2 For example, Kosseva (2013)3 reported that phenolic compounds are major contributors to the antioxidant capacity of common fruits and vegetables and their agrowastes. It has been claimed that polyphenolics from plants can alter glucose utilization in mammals, causing insulin-like effects.4 One therapeutic approach to treat diabetes is to retard the absorption of glucose via inhibition of enzymes, such as α-glucosidase, in the digestive organs. It has been confirmed that α-glucosidase activity in vitro can be inhibited by berry extracts, i.e. blueberry, blackcurrant, strawberry, and raspberry rich in polyphenols.4Strawberry (Fragaria × ananassa) is one of the most consumed berries. More than 50% of the cultivated areas of strawberry world production are located in Europe, with Poland, Serbia, Germany, Ukraine and Italy as the leading producers.5 Strawberry occupies the third position according to the importance and the production size in Serbia. In recent years, ‘Marmolada’ and ‘Clery’ are among several new introduced cultivars showing excellent agronomic results and becoming very popular based on their early growing season and excellent fruit quality.
Manufacturing of products derived from the strawberry generates high volumes of agro-waste which represents an environmental problem in the production areas that requires different technical waste management solutions. Sójka et al. (2013)6 reported that industrial strawberry press cake that remains after juice processing amounts to about 4% of the initial weight of raw material. Over the last years considerable emphasis has been put on recovery, recycling and upgrading of wastes that can be transformed into several different products like bio-fuels, multifunctional food ingredients, nutrients, food flavours, fodder, feed and operational supply like bio-adsorbents for waste water treatment.7 According to Bates et al. (2001)8 the pulp or press residue of high value fruits can be extracted with water or other solvents to yield extracts containing pigments, nutrients, nutraceuticals, essences or other useful by-products.
Based on these facts, the aim of the present work was to assess, for the first time, the phenolic composition and biological activity of pomaces obtained from two strawberry varieties with major commercial importance, cv. ‘Marmolada’ (SM) and cv. ‘Clery’ (SC). The prospects of utilization for food and health industries has been also outlined.
2. Methodology
2.1. Chemicals and instruments
Cyanidin-3-glucoside was from polyphenols (Sandnes, Norway), all standard phenolic acids and flavonoids, 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2′-azino-bis(3-ethyl benzothiazoline-6-sulfonic acid) diammonium salt (ABTS), 4-nitrophenil α-D-glucopyranoside, and α-glucosidase from Saccharomyces cerevisiae were from Sigma Chemicals Co. (St. Louis, MO, USA). Other used chemicals and solvents were of the highest analytical grade. Ultrapure water used was produced using an Elix 3 Millipore water purification system coupled to a Milli-Q module (model Advantage 10) (Millipore, Molsheim, France).Absorbances in spectrophotometrical assays were measured on UV-1800 spectrophotometer (Shimadzu, Kyoto, Japan). α-Glucosidase assay was measured using Infinite@M200 micro plate reader (Tecan, Grödig, Austria) and by using 96-well micro plates (Nunc, Roskilde, Denmark). Hydroxyl and superoxide anion radical antioxidant capacities were carried out on an ESR spectrometer from Bruker (Rheinstetten, Germany). For identification and quantification of phenolic acids and flavonoids samples were analyzed on Shimadzu Prominence chromatographic system, connected to the SPD-20AV UV/Vis detector (Shimadzu, Kyoto, Japan). The HPLC-DAD-ESI/MSn analyses were carried out on an Agilent HPLC 1100 series equipped with a PDA detector and a mass detector in series (Agilent Technologies, Waldbronn, Germany). The mass detector was an ion trap spectrometer (model G2445A) equipped with an electrospray ionization interface. Freeze-dryer, model Christ Alpha 2-4 LSC, was from Martin Christ, Osterode am Harz, Germany. High performance homogenizer (model Silent Crusher M) and shaker (model Unimax 1010) were from Heidolph Instruments GmbH, Kelheim, Germany. Centrifuge, model EBA 21, was from Hettich Zentrifugen, Tuttlingen, Germany.
2.2. Plant material
Strawberries (Fargaria × ananassa, cv. ‘Clery’ (SC) and ‘Marmolada’ (SM)) were grown by the conventional method and harvested by hand at the fully ripe stage on a farm Alfa RS (Lipolist, Serbia). All of the calyxes of strawberries were removed, fresh undamaged berries were washed and packed immediately, frozen and stored in a freezer at −20 °C until use.2.3. Sample preparation
Strawberry pomace was obtained by pressing the unfrozen berry fruits through a cheesecloth. The yields of pomace compared to the unfrozen fruits were 10.59 and 9.84% for SC and SM, respectively. Moisture contents in SC and SM pomaces were 92.30 and 91.79%, respectively. The extraction of (poly)phenolic substances from pomace was performed using 80% methanol aqueous solution with 0.05% acetic acid. Samples of pomace (20 g) were extracted in three steps: with 160 mL for 3 min on a high performance homogenizer and for 60 min on a laboratory shaker, and with additional 80 mL for 30 min. Two obtained extracts were combined, concentrated under reduced pressure and freeze-dried. The yields (Y) of the obtained extracts were: YSC = 10.27%, and YSM = 9.54%, respectively.2.4. Total phenolic content
Total phenolic contents in extracts were determined spectrophotometrically according to the Folin–Ciocalteu method.9 The results were expressed as mg of gallic acid equivalents per 100 g of strawberry pomace fresh weight/dry weight (mg GAE per 100 g FW; mg GAE per 100 g DW).2.5. Total flavonoid contents
Total flavonoids content was measured using assay developed by Zhishen et al. (1999).10 The flavonoids content was expressed as mg of rutin equivalents per 100 g of strawberry pomace fresh weight/dry weight (mg RE per 100 g FW; mg RE per 100 g DW).2.6. Total and monomeric anthocyanin contents
The total and monomeric anthocyanins content in extracts were estimated spectrophotometrically using the pH single and pH differential method.11 Anthocyanins were expressed in terms of cyanidin-3-glucoside equivalents per 100 g of strawberry pomace fresh weight per dry weight (mg CyGE per 100 g FW; mg CyGE per 100 g DW).2.7. Polyphenol analysis by chromatographic methods
For identification and quantification of polyphenols pomace extracts (100 mg) were extracted in polypropylene-capped tubes using 1.5 mL of 50% methanol for 1 h in ultrasonic bath, the mixture was placed in the dark at 4 °C for 24 h and then extracted for another hour in ultrasonic bath followed by centrifugation for 5 min at 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 945.75 × g. Supernatant was filtered through a 0.2 μm inorganic membrane filter (ANOTOP 10 plus, Whatman, Maidstone, UK) before injection into the HPLC system.
945.75 × g. Supernatant was filtered through a 0.2 μm inorganic membrane filter (ANOTOP 10 plus, Whatman, Maidstone, UK) before injection into the HPLC system.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) and acetonitrile were used as mobile phases A and B, respectively, with a flow rate of 1 mL min−1. The linear gradient started with 8% of solvent B, reaching 15% solvent B at 25 min, 22% at 55, and 40% at 60 min, which was maintained up to 70 min. Chromatograms were recorded at 520 nm. The ionization conditions were adjusted at 350 °C and 4 kV for capillary temperature and voltage, respectively. The nebulizer pressure and flow rate of nitrogen were 65.0 psi and 11 L min−1, respectively. The full-scan mass covered the range from m/z 100 up to m/z 1200. Collision induced fragmentation experiments were performed in the ion trap using helium as the collision gas, with voltage ramping cycles from 0.3 up to 2 V. Mass spectrometry data were acquired in the positive ionization mode. MSn was carried out in the automatic mode on the more abundant fragment ion in MS(n − 1).
1, v/v) and acetonitrile were used as mobile phases A and B, respectively, with a flow rate of 1 mL min−1. The linear gradient started with 8% of solvent B, reaching 15% solvent B at 25 min, 22% at 55, and 40% at 60 min, which was maintained up to 70 min. Chromatograms were recorded at 520 nm. The ionization conditions were adjusted at 350 °C and 4 kV for capillary temperature and voltage, respectively. The nebulizer pressure and flow rate of nitrogen were 65.0 psi and 11 L min−1, respectively. The full-scan mass covered the range from m/z 100 up to m/z 1200. Collision induced fragmentation experiments were performed in the ion trap using helium as the collision gas, with voltage ramping cycles from 0.3 up to 2 V. Mass spectrometry data were acquired in the positive ionization mode. MSn was carried out in the automatic mode on the more abundant fragment ion in MS(n − 1).For quantification experiments, the same HPLC system was used as for analysis of phenolic acids and flavonoids. Mobile phase, flow rate and linear gradient was the same as that for identification. Chromatograms were recorded at 520 nm and anthocyanins quantified using cyanidin-3-O-glucoside as standard.
2.8. Antioxidant capacity (AC) assays
Antioxidant capacity of strawberry pomaces was evaluated by O2˙−, ˙OH, DPPH˙ and ABTS+˙ assays. , EC50˙OH, ECDPPH˙50 and
, EC50˙OH, ECDPPH˙50 and 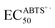 were defined as the concentration of an antioxidant extract which was required to quench 50% of the initial amount of O2˙−/˙OH/DPPH˙/ABTS+˙ radicals under the experimental conditions given. It was obtained by interpolation from linear regression analysis. EC50 values were also expressed as millimoles Trolox equivalent per gram of pomace extract dry weight (mmol TE g−1).
were defined as the concentration of an antioxidant extract which was required to quench 50% of the initial amount of O2˙−/˙OH/DPPH˙/ABTS+˙ radicals under the experimental conditions given. It was obtained by interpolation from linear regression analysis. EC50 values were also expressed as millimoles Trolox equivalent per gram of pomace extract dry weight (mmol TE g−1).
O2˙− and ˙OH antioxidant capacities were carried out using ESR spin trapping methods while DPPH˙ and ABTS+˙ antioxidant capacities were determined spectrophotometrically.
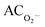 value was defined as:
value was defined as: 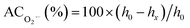 , where h0 and hx are the heights of the second peak in the ESR spectrum of DMPO/O2˙− spin adduct of the sample without and with antioxidant, respectively.
, where h0 and hx are the heights of the second peak in the ESR spectrum of DMPO/O2˙− spin adduct of the sample without and with antioxidant, respectively.2.9. Reducing power
The reducing power (RP) of strawberry pomaces was determined by the method of Oyaizu (1986).15 The sample concentration providing 0.5 of absorbance (ECRP50) was calculated from the graph of absorbance at 700 nm against extract concentration. ECRP50 value was also expressed as millimoles Trolox equivalent per gram of pomace extract dry weight (mmol TE g−1).2.10. α-Glucosidase inhibitory potential
α-Glucosidase inhibitory potential (α-GIP) was assessed using method reported by Tumbas Šaponjac et al. (2014).14 The absorbance of 4-nitrophenol released from 4-nitrophenyl α-D-glucopyranoside was measured at 405 nm and compared with that of the control to calculate the inhibitory activity. The sample concentration providing 50% inhibition of α-glucosidase enzyme activity (ECα-GIP50) was calculated from the graph of α-GIP (%) against extract concentration.2.11. Statistical analysis
All experiments were run in triplicate. The results represented are means ± standard deviation (±SD, n = 3). Statistical analyses were done by using Origin 7.0 SRO software package (OriginLab Corporation, Northampton, MA, USA, 1991–2002) and Microsoft Office Excel 2007 software. Significant differences were calculated by Student's t-test (p < 0.05, unless noted otherwise).3. Results and discussion
3.1. Polyphenols in strawberry pomaces
Two strawberry cultivars, ‘Clery’ and ‘Marmolada’, were used to obtained pomaces under laboratory conditions. Laboratory process yielded in somewhat higher percent of leftover (10.59 and 9.84% for SC and SM, respectively) compared to the one obtained in industry (4%).6 The reason for this could be lower amount of water remained in industrial pomace due to higher pressure applied in the industrial process. For example, Siles et al. (2013)16 reported that strawberry waste, provided from the manufacture by the ADESVA Technology Center of Huelva, had the moisture content of 77.92 ± 0.24%, which is lower than the ones obtained in this study for SC and SM laboratory pomaces (92.3 and 91.79%, respectively).Polyphenolic composition and quality in fruits depend on genetic (i.e. genus, species, cultivar/genotype) and environmental (i.e. fruit maturity, plant age, growing season, field location) factors. Del Pozo-Insfran et al. (2006)17 showed the appreciable effects of cultivar, harvest date, and production year on sugar, polyphenolics and antioxidant content in 22 strawberry lines. Strawberries are widely considered to be a good dietary source of vitamins and minerals and bioactive compounds, yet concentrations can vary greatly among cultivars and with postharvest handling conditions.5 The screening of the polyphenolic profiles of strawberry pomace was firstly carried out using spectrophotometrical assays and the results are presented in Table 1.
| Bioactive compounds | mg per 100 g FW | mg per 100 g DW | ||
|---|---|---|---|---|
| SC | SM | SC | SM | |
| a Data expressed as mean value of three replicate analysis ± SD.b Data expressed as mg GAE equivalents per 100 g of pomace FW/DW.c Data expressed as mg RE equivalents per 100 g of pomace FW/DW.d Data expressed as mg CyGE equivalents per 100 g of pomace FW/DW.e SC = ‘Clery’ pomace; SM = ‘Marmolada’ pomace.f Difference among values in the same row sharing the same unit is significant with *p < 0.05, **p < 0.01 and ***p < 0.001.g n.d. – not detected. | ||||
| Total polyphenolsb | 320.00 ± 22.06 | 313.65 ± 21.23 | 4155.84 ± 286.49 | 4073.38 ± 275.71 |
| Total flavonoidsc | 252.66 ± 5.59** | 285.53 ± 4.87** | 3281.30 ± 72.60** | 3708.18 ± 63.25** |
| Total anthocyaninsd | 34.06 ± 1.62* | 28.26 ± 1.73* | 442.34 ± 21.04* | 367.01 ± 22.47* |
| Monomeric anthocyaninsd | 28.29 ± 1.69* | 23.16 ± 1.45* | 367.40 ± 21.95* | 300.78 ± 18.83* |
| Protocatechuic acid | 4.79 ± 0.19*** | 150.93 ± 5.23** | 62.21 ± 2.98*** | 1838.31 ± 76.58*** |
| Gallic acid | 26.59 ± 1.10*** | 47.54 ± 1.73*** | 345.38 ± 15.26*** | 579.10 ± 21.37*** |
| Vanillic acid | 2.91 ± 0.12 | n.d. | 37.85 ± 1.12 | n.d. |
| Ellagic acid | 2.72 ± 0.09 | n.d. | 35.36 ± 1.68 | n.d. |
| Chlorogenic acid | 0.35 ± 0.01*** | 1.10 ± 0.04*** | 4.54 ± 0.13*** | 13.43 ± 0.47*** |
| p-Coumaric acid | 2.64 ± 0.09 | n.d. | 34.26 ± 1.17 | n.d. |
| Caffeic acid | 0.52 ± 0.02 | n.d. | 6.75 ± 0.27 | n.d. |
| Gentisic acid | 0.67 ± 0.03 | n.d. | 8.76 ± 0.32 | n.d. |
| Ferulic acid | 0.95 ± 0.03 | n.d. | 12.40 ± 0.42 | n.d. |
| Sinapic acid | 0.61 ± 0.02 | n.d. | 7.88 ± 0.34 | n.d. |
| Catechin | 19.56 ± 0.71*** | 135.19 ± 5.06*** | 254.01 ± 9.70*** | 1646.68 ± 62.41*** |
| Epicatechin | 1.07 ± 0.03 | n.d. | 13.93 ± 0.66 | n.d. |
| Epicatechin gallate | 5.65 ± 0.21 | n.d. | 73.36 ± 2.96 | n.d. |
| Rutin | 4.89 ± 0.18 | n.d. | 63.49 ± 2.65 | n.d. |
| Myricetin | 0.77 ± 0.03*** | 0.05 ± 0.00*** | 10.03 ± 0.25*** | 0.56 ± 0.02*** |
| Luteolin | 0.03 ± 0.00*** | 0.01 ± 0.00*** | 0.31 ± 0.01*** | 0.19 ± 0.01*** |
| Quercetin | 0.09 ± 0.00*** | 0.54 ± 0.02*** | 1.22 ± 0.05*** | 6.63 ± 0.23*** |
| Kaempferol | 0.08 ± 0.00*** | 0.13 ± 0.00*** | 0.99 ± 0.03*** | 1.56 ± 0.01*** |
Results presented in Table 1 showed higher values of polyphenolic compounds in SC pomace than in SM, but they did not differ significantly (p > 0.05). However, the contents of flavonoids (p < 0.01) and total and monomeric anthocyanins (p < 0.05) were significantly higher in SM pomace. Klopotek et al. (2005)18 reported that 17.5% of total phenols and 23.3% of total anthocyanins remain in the strawberry pomace, compared to their content in fresh strawberries. The results of Vulić et al. (2012)19 for total polyphenols (488.12 mg GAE per 100 g fresh pomace) and flavonoids (296.11 RE per 100 g fresh pomace) were somewhat higher, while total anthocyanins were lower (19.48 mg CyGE per 100 g fresh pomace) compared to the results obtained in this study. Krisch et al. (2009)20 found 13.61 and 17 μg per mg DW for polyphenols in water and methanol extracts of strawberry pomace, which is lower compared to our results when expressed in the same manner (31.17 and 32.87 μg per mg dry weight for SC and SM, respectively). Total polyphenols in SC and SM pomace were higher compared to other fruit and vegetable by-products (wastes from apple, kiwifruit, pink grapefruit and lettuce, white cabbage cut-offs, cauliflower cut-offs, broccoli stems) tested in the study of Wijngaard et al. (2009)21 ranging from 164 to 1867 mg GAE per 100 g DW.
Distribution of polyphenols in strawberry pomaces was further explored using HPLC (phenolic acids and flavonoids) and LC-MS (anthocyanins) analysis. Distribution of phenolic acids and flavonoids in SC and SM pomaces is presented in Table 1, while the characterization of anthocyanins is presented in Table 2 along with their HPLC-UV/Vis chromatograms presented on Fig. 1. Identification of anthocyanins was carried out using LC-MS data (retention times, molecular mass and MS2 ion fragments), while HPLC-UV/Vis chromatograms were used for quantification.
| Compound name | Rt (min) | Molecular mass [M + H]+ (m/z) | MS2 ion fragments (m/z) | mg per 100 g FW | mg per 100 g DW | ||
|---|---|---|---|---|---|---|---|
| SC | SM | SC | SM | ||||
| a Data expressed as mg CyGE per 100 g of pomace FW/DW and presented as mean value ± SD.b Pelargonidin-3-glucoside.c Pelargonidin-3-malonyl-glucoside.d SC = ‘Clery’ pomace; SM = ‘Marmolada’ pomace. | |||||||
| Plg-3-glcb | 33.52 | 433+ | 271 | 20.85 ± 0.71 | 17.82 ± 0.67 | 270.78 ± 10.24 | 231.43 ± 8.97 |
| Plg-3-malonyl-glcc | 48.45 | 519+ | 433, 271 | 8.16 ± 0.22 | 5.51 ± 0.23 | 106.03 ± 3.86 | 71.56 ± 2.15 |
 | ||
| Fig. 1 HPLC chromatograms of strawberry pomaces SC (a) and SM (b) recorded on 520 nm. Peak identification: 1 – pelargonidin-3-glucoside; 2 – pelargonidin-3-malonylglucoside. | ||
HPLC data showed significant (p < 0.001) differences in phenolic acids and flavonoids content between two strawberry pomaces. The sum of phenolic acids (2430.84 mg per 100 g) and flavonoids (1655.62 mg per 100 g) content in SM pomace were about 4-fold higher than in SC pomace (555.41 and 417.35 mg per 100 g, respectively). SM pomace contained high amounts of protocatechuic acid, catechin and gallic acid, while in the SC pomace the most abundant compounds were gallic acid and catechin.
Huang et al. (2012)22 detected series of phenolic acids (gallic, protocatechuic, p-hydroxybenzoic, caffeic, p-coumaric and ellagic acid), epigallocatechin, catechin, malvidin-3-glucoside, quercetrin, luteolin, gallocatechin, catechin gallate, cyanidin, and cinnamic acid in strawberries. Protocatechuic acid was detected in strawberries in the range from 0.4 to 1.28 mg per 100 g FW.23 In strawberry spreads the concentration of protocatechuic acid ranged from 0.06 to 0.11 mg per 100 g. Furthermore, protocatechuic acid and quercetin were found to be the most stable phenolics.24 Spontaneous degradation of anthocyanins to phenolic acids and aldehydes has been reported to occur under experimental conditions.25 This fact could explain higher contents of this compound in our samples, especially in SM. The concentration of quercetin in fresh strawberry in literature ranges from 0.3 to 5.3 mg per 100 g FW, kaempferol from undetectable to 0.9 mg per 100 g FW, while myricetin contents are approximately 100 mg per 100 g FW.5 Kosar et al. (2004)26 showed that the major compounds in strawberries were ellagic acid during the green stage, pelargonidin-3-glucoside and p-coumaric acid during the pink stage, and pelargonidin-3-glucoside and p-coumaric acid during the ripe stage. The content of ellagic acid in the strawberry cultivars was found to be between 0.22 and 1.19 mg per 100 g FW.27 In this study ellagic acid was found in lower amount in SC pomace, while in SM pomace it was not detected. Hilz et al. (2005)28 reported that the content of phenolic compounds like ellagic acid in pomace is lower than data reported for fresh strawberry, strawberry puree and juice, because they transfer to juice with other soluble substances. Strawberries are among frequently consumed foods with a relatively high amount of ellagic acid (responsible for >30% of total phenolics in strawberries). However, free ellagic acid levels are generally low, and their detection is the result of acid hydrolysis products of ellagitannin breakdown.29 Jaroslawska et al. (2011)30 determined strawberry pomace components including ellagic, p-coumaric, p-benzoic acids, quercetin, kaempferol and anthocyanins with contents of 95, 14, 4, 50, 60 and 30 mg per 100 g DW, respectively. Beside differences in cultivars and cultivation conditions, different sample preparation could also cause different results compared to the ones obtained in this study.
SC and SM strawberry pomaces had the same anthocyanin profile consisting of two pelargonidin glucosides. Their individual contents and their sum in SC (29.01 mg per 100 g) was significantly higher (p < 0.01) than in SM pomace (23.33 mg per 100 g). The dominant anthocyanin compound in both strawberry pomaces was pelargonidin-3-glucoside, also known as callistephin. The content of this anthocyanin accounted for more than 70% of total anthocyanins in both strawberry pomaces. According to Zhao (2013)5 this fact also stands for the strawberry flesh. Giampieri et al. (2012)31 also reported that pelargonidin-3-glucoside is the major anthocyanin in strawberries, independent from genetic and environmental factors and that glucose seems to be the most common substituting sugar in strawberry anthocyanins. The major pigments of wild strawberries are pelargonidin-3-glucoside and cyanidin-3-glucoside, where the amounts of pelargonidin-3-glucoside prevail 10 to even 20 times.5 However, in this study, no cyanidin glucosides were found in strawberry pomaces. The possible explanations for this could be that they were completely transferred to juice during pressing, or that strawberry anthocyanin pigments are less stable and that they were degraded during juice-processing.8
3.2. Biological activity of strawberry pomaces
In the present study a screening of biological activities of strawberry pomaces was carried out by defining their antioxidant capacities on O2˙− and ˙OH (Fig. 2a), DPPH˙ and ABTS˙+ (Fig. 2b), reducing power (Fig. 3a) and α-glucosidase inhibition potential (Fig. 3b).Reactive oxygen species, namely O2˙− and ˙OH, have been implicated as substances leading to cell damage. The reactivity of O2˙− itself may be too low to account for the damage observed in biological systems. However, many harmful effects of O2˙− are believed to be indirect, resulting from its conversion to ˙OH.32 Strawberry pomaces SC and SM demonstrated good antioxidant capacities in ˙OH and O2˙− assays, whereas the activity towards O2˙− was expressed in lower concentration range (0.02–1.5 mg mL−1) than towards ˙OH (1.0–7.0 mg mL−1). This could be explained by the fact that naturally occurring compounds remove different free radical species by employing different mechanisms. In this study, ˙OH are generated in Fenton reaction system, which includes the presence of Fe2+ ions, while O2˙− were generated chemically, with potassium superoxide and crown ether. Different interactions between reactants and compounds present in extracts are possible, e.g. chelation of Fe2+ ions, hydrogen donation, scavenging, reduction etc. AC˙OH and  of SM strawberry pomace was higher in the case of both free radical species. SM pomace expressed better activity than SC in all other tests used in this study as well. The difference between the ACDPPH˙, ACABTS+˙ and RP of SM and SC were not significantly different (p > 0.05), while α-GIP of SM was significantly higher (p < 0.05) than for SC.
of SM strawberry pomace was higher in the case of both free radical species. SM pomace expressed better activity than SC in all other tests used in this study as well. The difference between the ACDPPH˙, ACABTS+˙ and RP of SM and SC were not significantly different (p > 0.05), while α-GIP of SM was significantly higher (p < 0.05) than for SC.
The fact that SM pomace presented more powerful biological activity in all tests is in accordance with generally higher polyphenolic contents in this sample, suggesting that these compounds are involved in aforementioned activities. Furthermore, the major difference between SM and SC pomace is notably higher concentration of protocatechuic acid in SM. Catechol-type o-diphenols such as protocatechuic acid, show high antiradical activity towards DPPH radical, as they are readily converted to the corresponding o-quinones and further complex products. It was reported that protocatechuic acid balances the endogenous antioxidant system in pheochromocytoma cell line PC12, and in spleen and liver of aged rats.25 It was reported by Giampieri et al. (2012)31 that strawberries have a greater antioxidant capacity (2- to 11-fold) than apples, peaches, pears, grapes, tomatoes, oranges, and kiwifruit. According to Jaroslawska et al. (2011)30 strawberry pomace supplementation resulted in partial protection against the prooxidant effect of a diet in animals increasing integral antioxidant capacity in serum. By this, they further supported the assumption about strawberry pomace phenolics having a strong antioxidant potential.
Inhibition of α-glucosidase enzyme slows the elevation of blood sugar following a carbohydrate meal. Toeller (1994)33 reported similar α-glucosidase inhibition rates for plant polyphenolic extracts and acarbose, therapeutically used to control type 2 diabetes mellitus. Giampieri et al. (2012)31 showed that extracts from several strawberry cultivars exhibited a rather uniform inhibitory potential against α-glucosidase, and a moderate or low α-amylase inhibitory potential. Besides anthocyanins, caffeic acid derivatives and protocatechuic acid have been reported to be an effective α-glucosidase inhibitors.31,34 It has been demonstrated that oral administration of protocatechuic acid to streptozotocin-induced diabetic rats for 45 days prevented the increase in plasma glucose and glycosylated hemoglobin, as well as decreased insulin and hemoglobin levels in plasma.31
EC50 values expressed in terms of extract concentrations (mg mL−1) or Trolox equivalents (mmol TE g−1) for all tested biological activities are presented in Table 3.
| Assay | mg mL−1 | mmol TE g−1 | ||
|---|---|---|---|---|
| SC | SM | SC | SM | |
a The sample concentration providing 50% of: 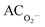 , AC˙OH, ACDPPH˙, ACABTS+˙, α-GIP and absorbance value of 0.5 for RP, expressed in mg mL−1 and mmol TE g−1.b SC = ‘Clery’ pomace; SM = ‘Marmolada’ pomace.c Mean values in the same row with the same unit are significantly different at the level **0.01 and ***0.001. , AC˙OH, ACDPPH˙, ACABTS+˙, α-GIP and absorbance value of 0.5 for RP, expressed in mg mL−1 and mmol TE g−1.b SC = ‘Clery’ pomace; SM = ‘Marmolada’ pomace.c Mean values in the same row with the same unit are significantly different at the level **0.01 and ***0.001. |
||||
| O2˙− | 0.24 ± 0.01*** | 0.12 ± 0.00*** | 1.13 ± 0.03*** | 0.55 ± 0.02*** |
| ˙OH | 3.38 ± 0.08*** | 2.77 ± 0.07*** | 31.88 ± 1.32** | 26.09 ± 1.16** |
| DPPH˙ | 0.11 ± 0.01 | 0.10 ± 0.01 | 2204.27 ± 89.12 | 2075.91 ± 92.55 |
| ABTS˙+ | 0.03 ± 0.00 | 0.03 ± 0.00 | 71.71 ± 2.55 | 68.34 ± 3.27 |
| α-GIP | 1.90 ± 0.04*** | 0.60 ± 0.01*** | — | — |
| RP | 1.24 ± 0.05 | 1.16 ± 0.04 | 69.28 ± 2.98 | 64.80 ± 3.04 |
Significantly higher (p < 0.001 or p < 0.01) antioxidant capacity of SM strawberry pomaces on O2˙− and ˙OH, and α-glucosidase inhibition potential as well, is evident also from the presented EC50 values. Lower EC50 values were found for SM in DPPH˙, ABTS˙+ and reducing power assays, as well. However, these differences were not significant at the level of 0.05.
Wang and Jiao (2000)35 determined that strawberries had the highest inhibition of active oxygen species (O2˙−, H2O2, OH˙, and O2) production among the several berry crops tested. Antioxidant capacity values against O2˙− of strawberry cultivars ranged from 40.4 to 51.4 μmol α-tocopherol per 10 g FW. Guo et al. (2003)36 found that the FRAP (ferric reducing antioxidant power) of strawberry pulp (3.29 mmol TE per 100 g wet weight) is higher than lemon pulp (0.91 mmol TE per 100 g wet weight). In the study of Wijngaard et al. (2009)21 apple pomace had 1435 mg TE per 100 g DW in FRAP assay while in DPPH assay it was 636 mg TE per 100 g DW, latter being lower than reported in this study for strawberry pomaces. Extracts of red grape pomaces, Vitis vinifera L. and Vitis labrusca L., showed lower antioxidant capacities against ABTS+˙ and DPPH˙ than SC and SM pomaces, ranging from 19.34 to 48.54 mmol TE per 100 g and from 188.02 to 505.52 mmol TE per 100 g, respectively.37 FRAP values of red grape pomaces were in the range from 117.79 to 249.46 mmol TE per 100 g. Significantly lower value in ABTS+˙ assay than in our study was reported by Müller et al. (2010)38 for strawberry puree (2.08 mmol TE per 100 g). According to the reports of Sójka et al. (2009)39 antioxidant capacities in DPPH˙ assay of blackcurrant pomace extracts were lower (from 3168.6 to 3820.8 μM TEAC g−1) than that of myricetin and quercetin (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 961 and 8615 μM TEAC g−1, respectively). These results have shown also considerably lower antioxidant capacity levels than our data. Many authors found strong influence of species and cultivar on antioxidant capacity of strawberries, whereas values differed from 2.5 to 3.5-fold, and that the total antioxidant activity is derived from the complex mixture of phytochemicals in the strawberry fruit, which can act in an additive and synergistic manner.5
961 and 8615 μM TEAC g−1, respectively). These results have shown also considerably lower antioxidant capacity levels than our data. Many authors found strong influence of species and cultivar on antioxidant capacity of strawberries, whereas values differed from 2.5 to 3.5-fold, and that the total antioxidant activity is derived from the complex mixture of phytochemicals in the strawberry fruit, which can act in an additive and synergistic manner.5
Boath et al. (2012)40 reported that black currant and rowanberry extracts effectively inhibit α-glucosidase with EC50 values of 20 and 30 μg GAE mL−1, respectively, which is lower than EC50 values for acarbose, chlorogenic acid and cyanidin-3-O-glucoside (40 μg mL−1, 300 μg mL−1, and 205 μg mL−1, respectively). In other study, Gironés-Vilaplana et al. (2014)41 reported that EC50 values of α-glucosidase inhibition for açaí and maqui berries were in range 0.33–2.14 mg mL−1. In our study strawberry pomaces were not so influential in inhibition of α-glucosidase. This is also supported by previous work of McDougall et al. (2005)4 that indicated low efficiency of strawberry extracts in α-glucosidase inhibition.
4. Conclusion
Strawberry pomace, a by-product from industrial fruit processing, constitutes a promissing source of polyphenolic compounds, including phenolic acids, non-coloured flavonoids and anthocyanins. Strawberry pomace possesses notable reactive oxygen and stable radicals antioxidant capacity, reducing power as well as moderate α-glucosidase inhibition potential. Taking into account that the literature lacks data on the properties of products derived from strawberry pomace, the results of this study may be of support when selecting suitable and alternative source for the preparation of high value products. However, further studies are needed to demonstrate the efficacy and safety of nutraceuticals recovered from strawberry pomaces and efficient purification steps are necessary before bioactive compounds can be used as natural food ingredients.Acknowledgements
The authors from the Faculty of Technology, University of Novi Sad, would like to thank the Ministry of Education, Science and Technological Development of Serbia, for financial support of this research (project no. TR 31044). The authors from CEBAS-CSIC would like to express their gratitude to the CONSOLIDER-INGENIO 2010 Research Project FUN-C-FOOD (CSD2007-00063). Part of this work was also possible through the Excellence in Research 04486/GERM/06 funds at CEBAS-CSIC.References
- D. Del Rio, A. Rodriguez-Mateos, J. P. E. Spencer, M. Tognolini, G. Borges and A. Crozier, Dietary (Poly)phenolics in Human Health: Structures, Bioavailability, and Evidence of Protective Effects Against Chronic Diseases, Antioxid. Redox Signaling, 2013, 18(14), 1818–1892 CrossRef CAS PubMed
.
- K. Aaby, D. Ekberg and G. Skrede, Characterization of Phenolic Compounds in Strawberry (Fragaria × ananassa) Fruits by Different HPLC Detectors and Contribution of Individual Compounds to Total Antioxidant Capacity, J. Agric. Food Chem., 2007, 55, 4395–4406 CrossRef CAS PubMed
.
- M. R. Kosseva, in Food Industry Wastes – Assessment and Recuperation of Commodities, ed. M. Kosseva and C. Webb, Elsevier, Academic Press, London, 2013, pp. 103–120 Search PubMed
.
- G. J. McDougall, F. Shpiro, P. Dobson, P. Smith, A. Blake and D. Stewart, Different Polyphenolic Components of Soft Fruits Inhibit α-Amylase and α-Glucosidase, J. Agric. Food Chem., 2005, 53, 2760–2766 CrossRef CAS PubMed
.
- Y. Zhao, in Berry fruit: value-added products for health promotion, CRC Press, Taylor & Francis Group, Boca Raton, FL, USA, 2007 Search PubMed
.
- M. Sójka, E. Klimczak, J. Macierzyński and K. Kołodziejczyk, Nutrient and polyphenolic composition of industrial strawberry press cake, Z. Lebensm.-Unters. Forsch., 2013, 237, 995–1007 Search PubMed
.
- G. Laufenberg, B. Kunz and M. Nystroem, Transformation of vegetable waste into value added products: (A) the upgrading concept; (B) practical implementations, Bioresour. Technol., 2003, 87(2), 167–198 CrossRef CAS
.
- R. P. Bates, J. R. Morris and P. G. Crandall, Principles and practices of small- and medium-scale fruit juice processing, FAO Agricultural Services Bulletin, Food and Agriculture Organization of The United Nations, Rome, Italy, 2001, vol. 146 Search PubMed
.
- V. L. Singleton, R. Orthofer and R. M. Lamuela-Raventos, in Methods in enzymology, oxidant and antioxidants (Part A), ed. L. Packer, Academic Press, San Diego, 1999, pp. 152–178 Search PubMed
.
- J. Zhishen, T. Mengcheng and W. Jianming, The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals, Food Chem., 1999, 64, 555–559 CrossRef CAS
.
- J. Lee, R. W. Durst and R. E. Wrolstad, Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method; Collaborative study, J. AOAC Int., 2006, 88(5), 1269–1279 Search PubMed
.
- S. Ðilas, Ž. Knez, D. Četojević-Simin, V. Tumbas, M. Škerget, J. Čanadanović-Brunet and G. Ćetković, In vitro antioxidant and antiproliferative activity of three rosemary (Rosmarinus officinalis L.) extract formulations, Int. J. Food Sci. Tech., 2012, 47, 2052–2062 CrossRef PubMed
.
- J. J. Vulić, T. N. Ćebović, J. M. Čanadanović-Brunet, G. S. Ćetković, V. M. Čanadanović, S. M. Djilas and V. Tumbas Šaponjac, In vivo and in vitro antioxidant effects of beetroot pomace extracts, J. Funct. Foods, 2014, 6, 168–175 CrossRef PubMed
.
- V. Tumbas Šaponjac, A. Gironés-Vilaplana, S. Djilas, P. Mena, G. Ćetković, D. A. Moreno, J. Čanadanović-Brunet, J. Vulić, S. Sajčić and M. Krunić, Anthocyanin profiles and biological properties of caneberry (Rubus spp.) press residues, J. Sci. Food Agric., 2014, 94, 2393–2400 CrossRef PubMed
.
- M. Oyaizu, Studies on product of browning reaction prepared from glucose amine, Jpn. J. Nutr., 1986, 44, 307–315 CrossRef CAS
.
- J. A. Siles, A. Serrano, A. Martín and M. A. Martín, Biomethanization of waste derived from strawberry processing: advantages of pretreatment, J. Cleaner Prod., 2013, 42, 190–197 CrossRef CAS PubMed
.
- D. Del Pozo-Insfran, C. E. Duncan, K. C. Yu, S. T. Talcott and C. K. Chandler, Polyphenolics, ascorbic acid, and soluble solids concentrations of strawberry cultivars and selections grown in a winter
annual hill production system, J. Am. Soc. Hortic. Sci., 2006, 131(1), 89–96 CAS
.
- Y. Klopotek, K. Otto and V. Bohm, Processing Strawberries to Different Products Alters Contents of Vitamin C, Total Phenolics, Total Anthocyanins, and Antioxidant Capacity, J. Agric. Food Chem., 2005, 53, 5640–5646 CrossRef CAS PubMed
.
- J. J. Vulić, V. T. Tumbas, S. M. Savatović, S. M. Đilas, G. S. Ćetković and J. M. Čanadanović-Brunet, Polyphenolic content and antioxidant activity of the four berry fruits pomace extracts, Acta Period. Technol., 2012, 42, 1–288 Search PubMed
.
- J. Krisch, L. Galgóczy, T. Papp and C. Vágvölgyi, Antimicrobial and antioxidant potential of waste products remaining after juice pressing, Ann. Fac. Eng. Hunedoara, 2009, VII(4), 131–134 Search PubMed
.
- H. H. Wijngaard, C. Rößle and N. Brunton, A survey of Irish fruit and vegetable waste and by-products as a source of polyphenolic antioxidants, Food Chem., 2009, 116, 202–207 CrossRef CAS PubMed
.
- W. Huang, H. Zhang, W. Liu and C. Li, Survey of antioxidant capacity and phenolic composition of blueberry, blackberry, and strawberry in Nanjing, J. Zhejiang Univ., Sci., B, 2012, 13(2), 94–102 CrossRef CAS PubMed
.
- P. Mattila and J. Kumpulainen, Determination of Free and Total Phenolic Acids in Plant-Derived Foods by HPLC with Diode-Array Detection, J. Agric. Food Chem., 2002, 50, 3660–3667 CrossRef CAS PubMed
.
- M. Kadivec, Š. Može Bornšek, T. Polak, L. Demšar, J. Hribar and T. Požrl, Phenolic Content of Strawberry Spreads during Processing and Storage, J. Agric. Food Chem., 2013, 61, 9220–9229 CrossRef CAS PubMed
.
- M. D'Archivio, B. Scazzocchio, C. Giovannini and R. Masella, in Polyphenols in Human Health and Disease, ed. R. R. Watson, V. R. Preedy and S. Zibadi, Academic Press, Elsevier, London, 2014, pp. 177–189 Search PubMed
.
- M. Kosar, E. Kafkas, S. Paydas and K. H. Baser, Phenolic composition of strawberry genotypes at different maturation stages, J. Agric. Food Chem., 2004, 52, 1586–1589 CrossRef CAS PubMed
.
- J. L. Maas, S. Y. Wang and G. J. Galetta, Evaluation of strawberry cultivars for ellagic acid content, Hortic. Sci., 1991, 26, 66–68 Search PubMed
.
- H. Hilz, E. J. Bakx, H. A. Schols and A. G. J. Voragen, Cell wall polysaccharides in black currants and bilberries – characterisation in berries, juice, and press cake, Carbohydr. Polym., 2005, 59, 477–488 CrossRef CAS PubMed
.
- S. Tulipani, B. Mezzetti, F. Capocasa, S. Bompadre, J. Beekwilder, C. H. R. de Vos, E. Capanoglu, A. Bovy and M. Battino, Antioxidants, Phenolic Compounds, and Nutritional Quality of Different Strawberry Genotypes, J. Agric. Food Chem., 2008, 56, 696–704 CrossRef CAS PubMed
.
- J. Jaroslawska, J. Juskiewicz, M. Wroblewska, A. Jurgonski, B. Krol and Z. Zdunczyk, Polyphenol-Rich Strawberry Pomace Reduces Serum and Liver Lipids and Alters Gastrointestinal Metabolite Formation in Fructose-Fed Rats, J. Nutr., 2011, 141(10), 1777–1783 CrossRef CAS PubMed
.
- F. Giampieri, S. Tulipani, J. M. Alvarez-Suarez, J. L. Quiles, B. Mezzetti and M. Battino, The strawberry: Composition, nutritional quality, and impact on human health, Nutrition, 2012, 28, 9–19 CrossRef CAS PubMed
.
- T. Okuno, H. Kawai, T. Hasegawa, H. Ueno and K. Nakamuro, Enhancement of hydroxyl radical formation from superoxide anion radical in the presence of organic selenium compounds, J. Health Sci., 2001, 47, 240–247 CrossRef CAS
.
- M. Toeller, α-Glucosidase inhibitors in diabetes: efficacy in NIDDM subjects, Eur. J. Clin. Invest., 1994, 24, 31–35 CrossRef PubMed
.
- G. J. McDougall, N. N. Kulkarni and D. Stewart, Current developments on the inhibitory effects of berry polyphenols on digestive enzymes, BioFactors, 2008, 34, 73–80 CrossRef
.
- S. Y. Wang and H. Jiao, Scavenging capacity of berry crops on superoxide radicals, hydrogen peroxide, hydroxyl radicals, and singlet oxygen, J. Agric. Food Chem., 2000, 48, 5677–5684 CrossRef CAS PubMed
.
- C. Guo, J. Yang, J. Wei, Y. Li, J. Xu and Y. Jiang, Antioxidant activities of peel, pulp and seed fractions of common fruits as determined by FRAP assay, Nutr. Res., 2003, 23, 1719–1726 CrossRef CAS PubMed
.
- I. I. Rockenbach, E. Rodrigues, L. V. Gonzaga, V. Caliari, M. I. Genovese, A. E. de Souza Schmidt Gonçalves and R. Fett, Phenolic compounds content and antioxidant activity in pomace from selected red grapes (Vitis vinifera L. and Vitis labrusca L.) widely produced in Brazil, Food Chem., 2011, 127, 174–179 CrossRef CAS PubMed
.
- L. Müller, S. Gnoyke, A. M. Popken and V. Böhm, Antioxidant capacity and related parameters of different fruit formulations, LWT--Food Sci. Technol., 2010, 43, 992–999 CrossRef PubMed
.
- M. Sójka, S. Guyot, K. Kołodziejczyk, B. Król and A. Baron, Composition of puri-fied phenolics preparations obtained from an extract of industrial blackcurrant (Ribes nigrum L.) pomace, J. Hortic. Sci. Biotechnol., 2009, 100–106 Search PubMed
, ISAFRUIT Special Issue.
- A. S. Boath, D. Stewart and G. J. McDougall, Berry components inhibit α-glucosidase in vitro: Synergies between acarbose and polyphenols from black currant and rowanberry, Food Chem., 2012, 135, 929–936 CrossRef CAS PubMed
.
- A. Gironés-Vilaplana, N. Baenas, D. Villaño, H. Speisky, C. García-Viguera and D. A. Moreno, Evaluation of Latin-American fruits rich in phytochemicals with biological effects, J. Funct. Foods, 2014, 7, 599–608 CrossRef PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra14296a |
| This journal is © The Royal Society of Chemistry 2015 |


