Molecular assembly of alkyl chain-grafted poly(L-lysine) tuned by backbone chain length and grafted alkyl chain†
Bo-Yu Chen,
Yun-Chiao Huang and
Jeng-Shiung Jan*
Department of Chemical Engineering, National Cheng Kung University, No. 1, University Rd., Tainan, Taiwan 70101. E-mail: jsjan@mail.ncku.edu.tw
First published on 23rd February 2015
Abstract
The preparation of alkyl chain-grafted poly(L-lysine) (PLL) vesicles with tunable molecular assembly by varying the polypeptide chain length and grafted alkyl chain under different solution conditions was investigated. These amphiphilic copolypeptides self-assembled to form vesicular nanostructures with average sizes between 60 and 400 nm under acidic and neutral conditions. The molecular packing of alkyl chain-grafted PLL was determined by the degree of alkyl chain substitution (DS) and grafted alkyl chain length. Upon increasing the DS and/or alkyl chain length, the chain conformation changed from random coils to a helical conformation, which accompanied the protonation/deprotonation of the amino group, leading to changes in the amphiphilic nature of the copolypeptides and subsequently the vesicular size. It was found that the vesicular size and chain conformation adopted by PLL were also influenced by the backbone chain length, depending on the DS and alkyl chain length. With the versatility of the synthesis strategy, it is expected that additional functionality can be incorporated onto these copolypeptides for specific purposes.
Introduction
Amphiphilic copolymers that can self-assemble to form nano-organized structures such as lamellae, micelles, and vesicles have been extensively studied because of their unique characteristics, distinct from low molecular weight surfactant molecules, and potential applications in many fields.1–8 Polymer vesicles, which have exhibited improved stability and superior membrane properties compared to liposomes,2,3 are the most interesting aggregates from materials science and biological perspectives. They are promising materials for mimics of biomembranes, nanobioreactors, carriers/encapsulants, and so on.3,5,8 Recently, polypeptide-based block or graft copolymers have received increasing attention because they not only possess the essential structures and functions of proteins but also exhibit biocompatibility/biodegradability and stimuli-responsiveness.9–22 Unlike conventional amphiphilic copolymers, the amphiphilic nature and self-assembled structures of the polypeptide-based block or graft copolymers can be tuned by changes in polypeptide secondary conformation.9,10,21,23 Incorporation of polypeptide segments into the copolymers provides significant advantages in controlling the structure and function of the as-formed assemblies and provides various functional groups for conjugating versatile ligands. While the study of polypeptide-based assemblies has focused on block copolymers, polypeptide-based assemblies formed by graft copolymers are another important subject of study, which has received much less attention.23–28Poly(L-lysine) (PLL) is an important building block, which has frequently been incorporated in graft copolymers. Graft copolymers comprised of PLL have been demonstrated to be promising materials for biomedical fields such as gene therapy, nonfouling coatings, and so forth.29–34 For example, the graft copolymers, PLL grafted with different lipid molecules, were used to complex with plasmid DNA for non-viral delivery of plasmid DNA.35,36 The study revealed that PLL grafted with lipids was effective gene carriers and the extent of substitution appeared to be critical for effective plasmid delivery. It is expected that the amphiphilic graft copolymers would form assemblies in solutions. However, only a few studies reported the self-assembly of the graft copolymers with PLL as the backbone.21,24,26,28,37 Due to the readily available conjugation strategy, the synthesis of graft copolymers with PLL as the backbone is much easier than that of block copolymers. It is possible that the amphiphilicity and self-assembly behaviour of the graft copolymers can be controlled by simply adjusting the side chain properties including the substitution degree and grafted chain length.
Previously, we have reported the synthesis and self-assembly of PLL grafted hexanoyl group and their evaluation as carriers/encapsulants for protein encapsulation.28 The experimental data revealed that the size of the as-formed vesicles can be tune by substitution degree and the increase in the substitution degree can facilitate the polypeptide chains to adopt more α-helical conformation. In this study, we further investigated the influence of the substitution degree, grafted alkyl chain length, and backbone chain length on the polypeptide chain conformation and their self-assembly behaviour through the use of dynamic light scattering (DLS), aqueous electrophoresis, circular dichroism (CD), transmission electron microscopy (TEM), and small angle X-ray scattering (SAXS). The influence of the hydrophobic interaction and subsequent chain conformational changes upon alkyl chain substitution on their amphiphilicity and molecular assembly was systematically studied. The size and molecular structure of these polypeptide assemblies at different solution conditions such as pH and ionic strength were also studied. The interplay between the hydrophobic interaction and chain conformational changes determined the self-assembled nanostructures of alkyl chain-grafted poly(L-lysine). It is worth mentioning that, to our knowledge, the influence of backbone chain length on the polypeptide chain conformation and their self-assembly behaviour has not been reported. Previous study reported that palmitoyl glycol chitosan can self-assembled to form vesicles and their size increased with the increase in chitosan molecular weight.38
Experimental section
Materials
THF (ACS Reagent, Merck) and diethyl ether (Anhydrous, ACS Reagent, J. T. Backer) were dried using Na metal (99.95%, in mineral oil, Aldrich). Hexane (ACS Reagent, ECHO) was dried using calcium hydride (95%, Aldrich). The amino acid, Nε-Z-L-lysine (∼99%, Z: carboxybenzyl), was used as received from Sigma-Aldrich, as well as bis(1,5-cyclooctadiene) nickel(0) (98+%), 2,2′-bipyridyl (99+%), propionyl anhydride, hexanoyl anhydride, decanoyl anhydride, and tetradecanoyl anhydride. Trifluoroacetic acid (TFA, 99%) was supplied by Alfa Aesar. Triphosgene (98%, Sigma-Aldrich) was used as received, as was HBr (33 wt% in acetic acid) from Fluka. Anhydrous methanol (99.9%) was purchased from Merck.Synthesis of alkyl chain-modified PLL
Poly(Z-L-lysine) (PZLL) was synthesized using the nickel initiator, 2,2′-bipyridyl-Ni(1,5-cyclooctadiene) (BpyNiCOD), according to the previous reports.9,20 PLL was obtained by deprotecting the Z group using HBr. PLL polypeptides were modified with propionyl, hexanoyl, decanoyl, and tetradecanoyl groups at 20 and 40% of lysine molar ratio. Specifically, 1 g of PLL was dissolved in 10 mL of anhydrous methanol and a designated amount of anhydride was added to the solution in a glove box. The resulting solution was left to stir at room temperature for 1 day. Then the solution was dialyzed against de-ionized (DI) water for three days using a cellulose membrane dialysis tube (Sigma, MWCO 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–14
000–14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mL−1) and freeze dried to yield the product. 1H NMR of alkyl chain-grafted PLL (CD3OD): δ = 0.90 (–CH2CH2(CH2)mCH3, 3H), 1.31 (–C(O)CH2CH2(CH2)mCH3, 2mH; NHCH(CH2CH2CH2CH2NH2)C(O)–, 2H), 1.53 (–NHCH(CH2CH2CH2CH2NHC(O)R)C(O)–, 2H), 1.60 (–NHCH(CH2CH2CH2CH2NH2)C(O)–, 2H; –NHCH(CH2CH2CH2CH2NHC(O)R)C(O)–, 2H), 1.77 (–NHCH(CH2CH2CH2CH2NH2)C(O)–, 2H; –NHCH(CH2CH2CH2CH2NHC(O)R)C(O)–, 2H; –C(O)CH2CH2(CH2)mCH3, 2H), 1.85–2.14 (–NHCH(CH2CH2CH2CH2NH2)C(O)–, 2H), 2.22 (–CH2CH2(CH2)mCH3, 2H), 2.98 (–NHCH(CH2CH2CH2CH2NH2)C(O)–, 2H), 3.14–3.29 (–NHCH(CH2CH2CH2CH2NHC(O)R)C(O)–, 2H), 3.96 (–NHCH(CH2CH2CH2CH2NH2)C(O)–, 1H; –NHCH(CH2CH2CH2CH2NHC(O)R)C(O)–, 1H).
000 g mL−1) and freeze dried to yield the product. 1H NMR of alkyl chain-grafted PLL (CD3OD): δ = 0.90 (–CH2CH2(CH2)mCH3, 3H), 1.31 (–C(O)CH2CH2(CH2)mCH3, 2mH; NHCH(CH2CH2CH2CH2NH2)C(O)–, 2H), 1.53 (–NHCH(CH2CH2CH2CH2NHC(O)R)C(O)–, 2H), 1.60 (–NHCH(CH2CH2CH2CH2NH2)C(O)–, 2H; –NHCH(CH2CH2CH2CH2NHC(O)R)C(O)–, 2H), 1.77 (–NHCH(CH2CH2CH2CH2NH2)C(O)–, 2H; –NHCH(CH2CH2CH2CH2NHC(O)R)C(O)–, 2H; –C(O)CH2CH2(CH2)mCH3, 2H), 1.85–2.14 (–NHCH(CH2CH2CH2CH2NH2)C(O)–, 2H), 2.22 (–CH2CH2(CH2)mCH3, 2H), 2.98 (–NHCH(CH2CH2CH2CH2NH2)C(O)–, 2H), 3.14–3.29 (–NHCH(CH2CH2CH2CH2NHC(O)R)C(O)–, 2H), 3.96 (–NHCH(CH2CH2CH2CH2NH2)C(O)–, 1H; –NHCH(CH2CH2CH2CH2NHC(O)R)C(O)–, 1H).
Preparation of polymeric vesicles
The polymeric vesicles were prepared via dialysis method. 5 mg of the copolypeptide was dissolved in 2 mL of methanol and the resultant solution was transferred to a cellulose membrane (Sigma, MWCO 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–14
000–14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mL−1) and dialyzed against 100 mL of PBS (pH 7.4, I = 0.01, 0.02, or 0.05 N). The buffer solution was changed every 30 min at the first three hours and at least two times the next 6 h. The as-prepared particle solutions were diluted to different polypeptide concentrations and then adjusted to desired pH value.
000 g mL−1) and dialyzed against 100 mL of PBS (pH 7.4, I = 0.01, 0.02, or 0.05 N). The buffer solution was changed every 30 min at the first three hours and at least two times the next 6 h. The as-prepared particle solutions were diluted to different polypeptide concentrations and then adjusted to desired pH value.
Critical aggregation concentration (cac) measurements
The pyrene solution (1.0 × 10−5 M) in acetone was added into the vials and the solvent was evaporated under nitrogen gas stream. The particle solutions were added into the vials and the final concentration of pyrene was 1.0 × 10−7 M. The resultant solutions were placed in a refrigerator overnight. The solutions were taken out from the refrigerator to let the temperature of the solutions reaching the room temperature. The emission spectra were recorded with the wavelength between 350 and 500 nm at an integration time of 1.0 s using a fluorescence spectrophotometer (Hitachi FL-4500, Japan). The cac values were determined from the change of the I385/I372 value of pyrene since the emissions of pyrene at 372 and 385 nm wavelengths are sensitive to the surrounding environment. The I372/I385 value did not change much at low concentrations and then increased linearly with further increasing concentration. Then the cac can be determined by intersecting the two straight lines.Characterization of alkyl chain-grafted PLL copolypeptides
Gel permeation chromatography (GPC) measurements of PZLL were performed using a Viscotek system equipped with two ViscoGEL I-Series columns (catalog number: I-MBLMW-3078 and I-MBHMW-3078, Viscotek) for efficient separation, eluted with 0.1 M LiBr in DMF at 55 °C. The eluent flow rate was 1 mL min−1. The samples were dissolved in DMF and stored in a refrigerator. For GPC measurements, the solutions were taken out from the refrigerator to let the temperature of the solutions reaching the room temperature. 1H NMR spectra were recorded at 300 MHz on a Mercury 300 Varian spectrometer. PZLL and alkyl chain-grafted PLL copolypeptides (∼5 mg mL−1) were dissolved in respective TFA-d1 and CD3OD for NMR measurements.Dynamic light scattering (DLS) and electrophoresis analyses
The hydrodynamic diameter (Dh), polydispersity index (PDI), and zeta potential of the self-assembled structures were measured using Malvern Zetasizer, NANO ZS (Malvern Instruments Limited, UK) equipped with a He–Ne laser (633 nm). The intensity of the scattered light was measured at a 173° scattering angle. The hydrodynamic diameter and PDI of the self-assembled structures were determined using Malvern Instruments Dynamic Light Scattering software. The particle solutions in PBS were adjusted to desired pH value using NaOH or HCl solutions. Each data point was obtained from the samples prepared in three different batches for DLS and zeta potential measurements.Transmission electron microscopy (TEM) analysis
TEM measurements were performed on a Hitachi H7500 microscope with a Tungsten lamp and an excitation voltage of 80 kV. Negative stained samples were prepared as follows. 10 μL of particle solution (pH 7.4, 0.01 N) was dropped on a carbon-coated copper grid. After the evaporation of the solvent, the phosphotungstic acid (PTA) solution (0.5 wt%, 10 μL) was dropped on the copper grid and the extra liquid was removed by filter paper. Then the grid was dried in air for about 1 day before TEM characterization.Small angle X-ray scattering (SAXS) analysis
SAXS measurements were performed using a Bruker diffractometer (NanoSTAR U System, Bruker AXS Gmbh, Karlsruhe, Germany). The background subtracted data were desmeared against the beam length profile of the source. Samples were measured in a 1 mm quartz capillary at 25 °C. The scattering wavevector q = 4πλ−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin
sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ, defined by the scattering angle θ and λ, was calibrated with a standard sample of silver behenate. SAXS data were subtracted with the scattering from the same solutions without the polymers, and corrected for incoming flux, sample thickness and electronic noise of the detector. For SAXS measurements, the particle solutions were concentrated to about 5 mg mL−1 of polypeptide concentration or higher by using ultrafiltration.
θ, defined by the scattering angle θ and λ, was calibrated with a standard sample of silver behenate. SAXS data were subtracted with the scattering from the same solutions without the polymers, and corrected for incoming flux, sample thickness and electronic noise of the detector. For SAXS measurements, the particle solutions were concentrated to about 5 mg mL−1 of polypeptide concentration or higher by using ultrafiltration.
Circular dichroism (CD) analysis
CD spectra were measured over the wavelength range between 190 and 260 nm using a 0.1 cm quartz cell on a JASCO J-815 spectrometer (JASCO Inc, Japan). The particle solutions (0.5 mg mL−1) in PBS were adjusted to desired pH value using 0.01 to 0.5 N NaOH or HCl solutions. The percentages of different secondary conformations in polypeptides were computed using CD-fit 4 software.39Results and discussion
Synthesis and characterization of amphiphilic copolypeptides
The amphiphilic graft copolypeptides were synthesized by partially substituted PLL side chain with various alkyl chains. Z-protected PLL (PZLL) was prepared via ring-opening polymerization of Z-L-lysine NCA initiated by BpyNiCOD at room temperature for 24 hours. The number-average molecular weights of the three PZLL derived from GPC were 79![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400, 35
400, 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 and 17
600 and 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 with narrow molecular weight distributions (Mw/Mn = 1.1–1.23) (Table S1†). The PZLL polypeptides were deprotected by HBr to get PLL, denoted as K300, K130, and K60. Then the PLL was reacted with alkyl anhydride in anhydrous methanol as shown in Scheme 1 and the hydrogen atoms on the amino group of PLL were replaced by the alkyl chains. The four anhydrides, which are propionyl, hexanoyl, decanoyl, and tetradecanoyl anhydrides, were selected for the substitution reaction. The synthesis of alkyl chain-grafted PLL with different degrees of substitution can be achieved at the feed molar ratio of the anhydride to lysine to be 20 and 40%. The denotation of the graft copolypeptides was based on the PLL chain length, alkyl chain and substitution degree as shown in Table 1. For example, K130-g-Dec0.2 represents 20 mol% of decanoyl anhydride/lysine feed molar ratio was used for the grafting of decanoyl group onto the K130.
400 with narrow molecular weight distributions (Mw/Mn = 1.1–1.23) (Table S1†). The PZLL polypeptides were deprotected by HBr to get PLL, denoted as K300, K130, and K60. Then the PLL was reacted with alkyl anhydride in anhydrous methanol as shown in Scheme 1 and the hydrogen atoms on the amino group of PLL were replaced by the alkyl chains. The four anhydrides, which are propionyl, hexanoyl, decanoyl, and tetradecanoyl anhydrides, were selected for the substitution reaction. The synthesis of alkyl chain-grafted PLL with different degrees of substitution can be achieved at the feed molar ratio of the anhydride to lysine to be 20 and 40%. The denotation of the graft copolypeptides was based on the PLL chain length, alkyl chain and substitution degree as shown in Table 1. For example, K130-g-Dec0.2 represents 20 mol% of decanoyl anhydride/lysine feed molar ratio was used for the grafting of decanoyl group onto the K130.
| Sample code | Chain length of alkyl chain | Feed ratio (%) | Obtained DS (%) | Grafting efficiency (%) |
|---|---|---|---|---|
| K300-g-Dec0.2 | 10 | 20 | 16.9 | 84.5 |
| K300-g-Dec0.4 | 10 | 40 | 34.3 | 85.7 |
| K130-g-Prop0.2 | 3 | 20 | 18.2 | 91.0 |
| K130-g-Hexa0.2 | 6 | 20 | 17.6 | 88.0 |
| K130-g-Dec0.2 | 10 | 20 | 16.2 | 81.0 |
| K130-g-Dec0.4 | 10 | 40 | 33.5 | 83.7 |
| K130-g-Tetra0.2 | 14 | 20 | 15.0 | 75.0 |
| K60-g-Dec0.2 | 10 | 20 | 16.2 | 81.0 |
| K60-g-Dec0.4 | 10 | 40 | 30.9 | 77.2 |
| K60-g-Tetra0.2 | 14 | 20 | 17.0 | 85.0 |
The molecular structure and 1H NMR spectra of alkyl chain-grafted PLL in CD3OD were shown in Fig. 1 and S2.† The alkyl chain substitution was found to replace some of the amino groups of PLL, evidenced by the presence of all chemical shifts of the protons for alkyl chain-grafted PLL. The degrees of substitution (DS) for all of the samples were determined by the ε protons with or without alkyl chain substitution based on the following equation and the resultant grafting yields were shown in Table 1.
| DS = (integrated area at 3.10–3.20, (CH2)3CH2NHCO–)/[(integrated area at 2.88–3.03, (CH2)3CH2NH2) + (integrated area at 3.10–3.20, (CH2)3CH2NHCO–)] |
 | ||
| Fig. 1 1H NMR spectra of (a) K130-g-Pro0.2, (b) K130-g-Hexa0.2, (c) K130-g-Dec0.2, (d) K130-g-Dec0.4, and (e) K130-g-Tetra0.2 graft copolypeptides. | ||
As shown in Table 1, it was found that the grafting efficiencies were calculated to range between 75 and 91%. For the preparation of the decanoyl chain-grafted PLL (PLL-g-Dec) at the anhydride to lysine feed molar ratio higher than 60% and the tetradecanoyl chain-grafted PLL (PLL-g-Tetra) at the anhydride to lysine feed molar ratio higher than 40%, the substitution efficiencies were found to be lower than 70%.
Self-assembly of amphiphilic copolypeptides
The graft copolypeptides composed of hydrophobic alkyl chain and hydrophilic PLL backbone can self-assemble to form supramolecular structures by dialysis method. Using dialysis method, the alkyl chain-grafted PLL was dissolved in methanol and dialyzed against PBS (pH = 7.40, I = 0.01 N). The effects of PLL chain length, DS, alkyl chain length and solution condition on the self-assembly of alkyl chain-grafted PLL were investigated. The critical aggregation concentration (cac) was first characterized using the fluorescence probe technique. Pyrene was commonly chosen as the fluorescence probe to monitor the aggregation behaviour of surfactants and amphiphilic copolymers due to its emission of fluorescence in a hydrophobic environment formed in aqueous solution. In this study, the cac values were determined from the change of the I385/I372 value of pyrene by intersecting the two straight lines (Fig. S2 and S3†). As shown in Table 2, the cac values were found to depend on PLL chain length, alkyl chain length, and DS. For the graft copolypeptides with comparable PLL chain length, as expected, the increase of DS and/or alkyl chain length would result in the decrease of cac value, consistent with our previous studies.21,28 It is known that the increase of DS and/or alkyl chain length would lead to the increase in the hydrophobicity of the graft copolypeptides and subsequently the polypeptide assemblies would dissociate at lower concentration. Moreover, for the graft copolypeptides with comparable DS and alkyl chain length, it was found that the decrease of PLL chain length would result in the decrease of cac values.| Sample code | cac × 10−2 (mg mL−1) | pH 4.68, I = 0.01 N | pH 7.40, I = 0.01 N | ||||
|---|---|---|---|---|---|---|---|
| Dh (nm) | PDI | Zeta potential (mV) | Dh (nm) | PDI | Zeta potential (mV) | ||
| K300-g-Dec0.2 | 5.2 | 325 ± 14.4 | 0.33 ± 0.02 | 30.4 ± 0.5 | 181 ± 5.3 | 0.39 ± 0.01 | 12.4 ± 1.5 |
| K300-g-Dec0.4 | 3.1 | 267 ± 6.5 | 0.35 ± 0.12 | 30.3 ± 0.2 | 74 ± 2.6 | 0.08 ± 0.01 | 11.0 ± 0.4 |
| K130-g-Hexa0.2 | 16.3 | 401 ± 10.5 | 0.24 ± 0.04 | 44.5 ± 1.8 | 330 ± 9.0 | 0.21 ± 0.01 | 16.3 ± 0.4 |
| K130-g-Dec0.2 | 3.5 | 277 ± 12.1 | 0.21 ± 0.04 | 28.7 ± 3.6 | 179 ± 2.7 | 0.22 ± 0.02 | 12.0 ± 0.9 |
| K130-g-Dec0.4 | 1.7 | 200 ± 7.5 | 0.27 ± 0.01 | 35.7 ± 2.4 | 96 ± 3.6 | 0.06 ± 0.003 | 11.7 ± 0.4 |
| K130-g-Tetra0.2 | 1.1 | 103 ± 4.2 | 0.29 ± 0.03 | 16.4 ± 2.3 | 70 ± 4.4 | 0.24 ± 0.04 | 5.2 ± 1.5 |
| K60-g-Dec0.2 | 2.6 | 279 ± 15.8 | 0.40 ± 0.04 | 42.1 ± 2.3 | 224 ± 4.5 | 0.29 ± 0.05 | 13.1 ± 1.1 |
| K60-g-Dec0.4 | 1.2 | 204 ± 11.8 | 0.22 ± 0.02 | 40.8 ± 0.7 | 120 ± 4.2 | 0.08 ± 0.01 | 12.3 ± 0.4 |
| K60-g-Tetra0.2 | 0.9 | 96 ± 6.9 | 0.44 ± 0.10 | 14.6 ± 2.2 | 72 ± 9.3 | 0.24 ± 0.01 | 6.2 ± 0.4 |
The average hydrodynamic diameters (or sizes), Dh, of the self-assembled structures at pH 7.4 were measured by DLS. As shown in Table 2, DLS analysis revealed that these resultant assemblies exhibited monomodal distributions with their polydispersity index (PDI) values mostly smaller than 0.25 at neutral condition (Fig. 2a and b and S4†). K130-g-Prop0.2 graft copolypeptide cannot self-assemble in solution due to the low hydrophobic interaction. The sizes of these assemblies are measured to range between 60 and 350 nm, depending on the PLL chain length, alkyl chain length, and DS (Table 2). It is worth to note that their sizes did not change upon diluting or concentrating the sample solutions. For the graft copolypeptides with comparable PLL chain length, the increase of alkyl chain length and/or DS would result in the decrease in the sizes of the assemblies, consistent with our previous studies.21,28 Moreover, for PLL-g-Dec graft copolypeptides, the increase of DS led to the formation of assemblies with narrower size distributions, evidenced by the PDI values for PLL-g-Dec0.4 smaller than 0.1. For PLL-g-Dec graft copolypeptides, it was found that the decrease of PLL chain length would result in the increase in the sizes of the assemblies. The size of K130-g-Tetra0.2 assemblies at neutral condition was comparable with that of K60-g-Tetra0.2 assemblies. Previous study reported the self-assembly behaviour of acylated carboxymethyl chitosan can be manipulated by varying the substitution degree and grafted chain length.40 In this study, the results also clearly indicated that hydrophobic modification of hydrophilic PLL by using different alkyl chain lengths would have significant effect on their self-assembly behaviour. Zeta potential measurements revealed that these assemblies carried positive charge due to the presence of amino groups, evidenced by the positive zeta potential values. It was found that the zeta potential decreased slightly with the increase of alkyl chain length and/or DS. For the preparation of polypeptide assemblies using dialysis method, methanol was replaced by ethanol to dissolve the graft copolypeptides, followed by dialyzing against PBS (pH = 7.40, I = 0.01 N). DLS analysis revealed that these resultant assemblies exhibited monomodal distributions and their PDI values were mostly smaller than 0.25 at neutral condition (Table 3). As shown in Table 3, DLS analysis showed that the assemblies formed by the K300-g-Dec0.4, K130-g-Tetra0.2, and K60-g-Tetra0.2 graft copolypeptides also exhibited comparable sizes and zeta potential values as compared to those prepared by using methanol as the solvent. Rather, the K300-g-Dec0.2 assemblies exhibited the increase in size (from 181 to 264 nm) and size distribution as the solvent was switched from methanol to ethanol.
| Sample code | pH 7.40, I = 0.01 N, ethanol | ||
|---|---|---|---|
| Dh (nm) | PDI | Zeta potential (mV) | |
| K300-g-Dec0.2 | 264 ± 12.8 | 0.42 ± 0.06 | 14.1 ± 1.7 |
| K300-g-Dec0.4 | 73 ± 2.2 | 0.14 ± 0.02 | 12.5 ± 0.4 |
| K130-g-Tetra0.2 | 68 ± 2.0 | 0.19 ± 0.02 | 8.1 ± 1.3 |
| K60-g-Tetra0.2 | 64 ± 1.5 | 0.23 ± 0.01 | 9.5 ± 1.9 |
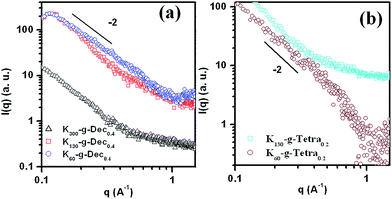
The self-assembled structures formed by these graft copolypeptides in PBS (pH = 7.40, I = 0.01 N) were characterized by TEM and SAXS analyses. These self-assembled structures were stained by PTA for TEM analysis. The results showed that K300-g-Dec0.2, K130-g-Hexa0.2, K130-g-Dec0.2, and K60-g-Dec0.2 graft copolypeptides self-assembled to form vesicles, evidenced by the observed ring structures (Fig. 3a and b and S5a†). Rather TEM images of other assemblies showed the observed dense cores, which could be attributed to the PTA staining (Fig. 3c and d, S5b and c†). The sizes of these assemblies determined by TEM characterization were found to be comparable with those by DLS analysis. In order to characterize the molecular packing architecture of these assemblies, small-angle X-ray scattering (SAXS) analysis was performed on PLL-g-Dec0.4 and PLL-g-Tetra0.2 assemblies. It is worth to note that the particle solutions were concentrated to about 5 mg mL−1 of polypeptide concentration or higher using ultrafiltration. SAXS spectra of these assemblies showed the scattering intensity (I) with characteristic I(q) ∝ q−2 at low scattering vectors (q) (Fig. 4), indicating the formation of vesicular structures.41 The results revealed that these alkyl chain-grafted PLL copolypeptides self-assembled to form vesicles (Scheme 1).
 | ||
| Fig. 3 TEM images of (a) K130-g-Hexa0.2, (b) K130-g-Dec0.2, (c) K130-g-Dec0.4, and (d) K130-g-Tetra0.2 assemblies at pH 7.4 (I = 0.01 N). | ||
The vesicular sizes can also be regulated by changing the solution condition such as the pH and ionic strength. As shown in Table 2, the vesicular sizes increased upon decreasing the solution pH from 7.4 to 4.68. The increase of the vesicular sizes is due to the increase in the degree of protonation for the amino group on PLL side chain, evidenced by the increase of their zeta potential. The increase in the degree of protonation for the amino group would cause the increase in the fraction of the hydrophilic segments and the repulsion between charged segments, and subsequently lead to the swelling of the vesicles. It was found that the vesicles started to aggregate upon adjusting the solution pH to basic due to the decrease in the degree of protonation for the amino group. The influence of ionic strength on their self-assembly was also studied. As shown in Fig. 2c, the size of K300-g-Dec0.2 vesicles decreased from 209 nm to 120 nm as the ionic strength increased from 0.005 to 0.02 N at neutral condition. On the contrary, The K300-g-Dec0.4 vesicular size increased from 78 nm to 156 nm as the ionic strength increased from 0.005 to 0.02 N. It can be attributed that the vesicles formed by K300-g-Dec0.2 graft copolypeptide were much less compact due to the relatively low alkyl chain grafting ratio as comparing with those form by K300-g-Dec0.4 graft copolypeptide. Consequently, the increase of ionic strength would lead to the formation of more compact assemblies due to charge screening effect. However, for K300-g-Dec0.4 vesicles, the rigid polypeptide segments would self-assemble to form bilayers with lower curvature upon increasing ionic strength.
Chain conformation of amphiphilic copolypeptides
The influence of alkyl chain length, DS, and PLL chain length on the secondary conformation adopted by the graft copolypeptides at different solution conditions was investigated. The sample solutions (0.5 mg mL−1) were prepared in PBS (pH 7.4) and then adjusted to acidic (pH 4.68) and basic (pH 10.0) conditions for CD measurements, respectively. It is worth noting that the polypeptide concentrations were well above their cac values. The percentages of different secondary conformations adopted by these graft copolypeptides were computed by using a software CD-fit4 by fitting a theoretical curve to the spectra (Table 4).39 At pH 4.68, the CD spectra for all graft copolypeptides exhibited a minimum at 197–199 nm and a maximum at 215–217 nm, revealing that these graft copolypeptides at acidic condition mainly adopted random coil conformation. At pH 7.4, the CD spectra of the alkyl chain-grafted PLL changed from the well-known doubly inflected curves to the ones with double minima upon increasing the DS or alkyl chain length (Fig. 5a and b), suggesting that the polypeptide chains underwent a coil-to-helix transition upon grafting alkyl chains. The percentages of helical conformation adopted by PLL-g-Dec0.4 graft copolypeptides at neutral condition increased with the increase in PLL chain length (Fig. S6c†) and rather PLL-g-Dec0.2 graft copolypeptides adopted comparable secondary conformation. The results supported that the increase of the vesicular sizes upon decreasing the solution pH from 7.4 to 4.68 due to the conformational changes from helix to coil and simultaneously the increase in the degree of protonation. At pH 10.0, the PLL-g-Dec and PLL-g-Tetra graft copolypeptides adopted mainly a mixture of α-helical and β-sheet/turn conformations, revealing that the polypeptide chains underwent a helix-to-sheet/turn transition upon changing the solution from neutral to basic condition (Fig. 5c and d, S6a and b†).| Sample code | pH | Random coil (%) | α-Helix (%) | β-Sheet (%) | β-Turn (%) |
|---|---|---|---|---|---|
| K300 | 4.68 | 95.02 | 5.0 | — | — |
| 7.40 | 93.2 | 6.8 | — | — | |
| 10.0 | 27.3 | 72.7 | — | — | |
| K300-g-Dec0.2 | 4.68 | 93.4 | 6.6 | — | — |
| 7.40 | 77.5 | 22.5 | — | — | |
| 10.0 | — | 39.4 | 49.0 | 11.6 | |
| K300-g-Dec0.4 | 4.68 | 80.5 | 19.5 | — | — |
| 7.40 | 39.9 | 60.1 | — | — | |
| 10.0 | — | 28.7 | 42.5 | 28.8 | |
| K130 | 4.68 | 91.2 | 8.8 | — | — |
| 7.40 | 92.3 | 7.7 | — | — | |
| 10.0 | 26.2 | 73.8 | — | — | |
| K130-g-Hexa0.2 | 4.68 | 94.7 | 5.3 | — | — |
| 7.40 | 91.1 | 8.9 | — | — | |
| 10.0 | 31.7 | 66.3 | — | 2.0 | |
| K130-g-Dec0.2 | 4.68 | 92.4 | 7.6 | — | — |
| 7.40 | 78.2 | 21.8 | — | — | |
| 10.0 | 2.5 | 33.4 | 46.3 | 17.7 | |
| K130-g-Dec0.4 | 4.68 | 82.2 | 17.8 | — | — |
| 7.40 | 55.8 | 44.2 | — | — | |
| 10.0 | — | 29.7 | 43.8 | 26.5 | |
| K130-g-Tetra0.2 | 4.68 | 76.6 | 23.4 | — | — |
| 7.40 | 27.5 | 72.5 | — | — | |
| 10.0 | — | 45.8 | 44.2 | 10.0 | |
| K60 | 4.68 | 91.7 | 8.3 | — | — |
| 7.40 | 90.8 | 9.2 | — | — | |
| 10.0 | 21.2 | 78.8 | — | — | |
| K60-g-Dec0.2 | 4.68 | 90.6 | 9.4 | — | — |
| 7.40 | 79.2 | 20.8 | — | — | |
| 10.0 | — | 43.0 | 43.4 | 13.6 | |
| K60-g-Dec0.4 | 4.68 | 84.3 | 15.7 | — | — |
| 7.40 | 63.5 | 36.5 | — | — | |
| 10.0 | — | 24.6 | 51.5 | 23.9 | |
| K60-g-Tetra0.2 | 4.68 | 76.4 | 23.6 | — | — |
| 7.40 | 28.1 | 71.9 | — | — | |
| 10.0 | — | 23.0 | 60.8 | 16.2 |
 | ||
| Fig. 5 CD spectra of (a) alkyl chain-grafted K130 and (b) alkyl chain-grafted K60 at pH 7.4 (I = 0.01 N) and CD spectra of (c) K130-g-Dec0.4 and (d) K130-g-Tetra0.2 at different pH values. | ||
For comparison, the CD spectra of K300, K130, and K60 revealed that the PLL chains adopted mainly random coil conformation at acidic and neutral conditions and underwent a coil-to-helix transition upon adjusting the solution pH to basic condition due to the decrease in the degree of protonation for the amino group. Previously, we have shown that the increase in the degree of hexanoyl substitution on PLL resulted in the increase of relative contents of α-helical conformation adopted by the PLL-g-Hexa.28 However, the hexanoyl substitution did not lead to the conformational transition. This study revealed that the PLL chains grafted with longer alkyl chains and higher DS can undergo conformational transitions. Also, PLL chain length would influence the secondary conformation adopted by the graft copolypeptides.
It is well known that PLL chains would undergo a coil-to-helix transition upon increasing the solution pH to basic condition at ambient temperature and a helix-to-sheet transition at the temperature higher than 55 °C in alkaline media. It has been previously reported that a short PLL chain conjugated with a polymer block can facilitate the coil-to-helix transition upon increasing pH and the helix-to-sheet transition upon heating.42,43 We have reported that the confinement of PLL-based block or graft copolypeptide chains in the assembled structures can facilitate the lysine segments to undergo conformational transition.21,44,45 In this study, the results revealed that the alkyl chain substitution as well as the confined environment due to self-assembly can facilitate the graft copolypeptides to undergo conformational transitions.
Influence of amphiphilicity and chain conformation on self-assembly
It has already been reported that the self-assembly of polypeptide-based copolymers are significantly relevant to their conformation and the curvature of the hydrophilic–hydrophobic interface.3,5,9,11 In this study, the self-assembled amphiphilic graft copolypeptides were significantly influenced by their amphiphilicity, which was correlated with the PLL chain conformation. The protonation of the amino group can lead to the PLL chains carrying positive charge and adopting random coil conformation, which is flexible and hydrophilic in nature. In contrast, the PLL chains that adopted the unionized, ordered conformation such as α-helices or β-sheets/turns would result in the decrease in the flexibility and hydrophilicity of the polypeptide chains. DLS analysis showed that the sizes of the vesicles decreased with the increase in DS and/or alkyl chain length as well as PLL molecular weight. The results clearly revealed that the increase in hydrophobic interaction due to the increase in the grafted alkyl chain length and/or DS resulted that the polypeptide chains were embedded in much confined environment, which consequently facilitated the polypeptide chains adopted more un-ionized, ordered conformation and smaller vesicular sizes. It was found that the vesicular size decreased with the increase in PLL molecular weight. For PLL-g-Dec0.4 vesicles, the vesicular size decreased with the increase in PLL molecular weight, which can be attributed mainly the increase in the percentages of helical conformation. The zeta potential measurements supported that the vesicles carried less positive charge upon increasing alkyl chain length and/or DS due to adapting more helical conformation. SAXS and TEM analyses revealed that alkyl chain-grafted PLL copolypeptides self-assembled to form vesicles. Since the graft copolypeptides can be regarded as “comb-shaped” molecular structures, the PLL backbones must be parallel to the interface instead of oriented orthogonally and the PLL segments adopted ordered conformation would probably reside above and below the hydrophobic regime as shown in Scheme 1. Based on the results, it is hard to determine whether the membrane has a bilayered or an interdigitated structure.Conclusion
In this work, we demonstrated that the alkyl chain-grafted PLL vesicles with tunable molecular assembly can be prepared by varying polypeptide chain length, grafted alkyl chain, and DS. The cac and size of the vesicles were found to decrease with the increase of the DS and/or grafted alkyl chain length. The chain conformational changes from random coils to helical conformation, which was accompanied with the protonation/deprotonation of the amino group, upon increasing the DS and/or alkyl chain length, resulting in the changes in the amphiphilic nature and size of these vesicles. The interplay between the hydrophobic interaction and chain conformational changes upon alkyl chain substitution and varying solution conditions determined the molecular assembly of alkyl chain-grafted PLL. It was found that the vesicular size decreased with the increase in PLL molecular weight. At given DS, the size of the polypeptide vesicles decreased with the increase of polypeptide chain length. With versatility of this synthesis strategy, additional functionality can be incorporated onto these amphiphilic copolypeptides, which can allow them to be useful as targeted drug carriers, nanoencapsulants in the biomedical fields.Acknowledgements
J.-S. J. acknowledges funding support from Ministry of Science and Technology grant MOST102-2221-E-006-267. J.-S. J. also acknowledges S. S.-S. Wang for access to the circular dichroism.Notes and references
- S. Förster and M. Antonietti, Adv. Mater., 1998, 10, 195–217 CrossRef.
- D. E. Discher and A. Eisenberg, Science, 2002, 297, 967–973 CrossRef CAS PubMed.
- K. Kita-Tokarczyk, J. Grumelard, T. Haefele and W. Meier, Polymer, 2005, 46, 3540–3563 CrossRef CAS PubMed.
- A. Blanazs, S. P. Armes and A. J. Ryan, Macromol. Rapid Commun., 2009, 30, 267–277 CrossRef CAS PubMed.
- A. Carlsen and S. Lecommandoux, Curr. Opin. Colloid Interface Sci., 2009, 14, 329–339 CrossRef CAS PubMed.
- R. P. Brinkhuis, F. P. J. T. Rutjes and J. C. M. van Hest, Polym. Chem., 2011, 2, 1449–1462 RSC.
- C. He, X. Zhuang, Z. Tang, H. Tian and X. Chen, Adv. Healthcare Mater., 2012, 1, 48–78 CrossRef CAS PubMed.
- L. Zhao, N. Li, K. Wang, C. Shi, L. Zhang and Y. Luan, Biomaterials, 2014, 35, 1284–1301 CrossRef CAS PubMed.
- E. G. Bellomo, M. D. Wyrsta, L. Pakstis, D. J. Pochan and T. J. Deming, Nature Mater., 2004, 3, 244–248 CrossRef CAS PubMed.
- J. Rodríguez-Hernández and S. Lecommandoux, J. Am. Chem. Soc., 2005, 127, 2026–2027 CrossRef PubMed.
- F. Chécot, A. Brûlet, J. Oberdisse, Y. Gnanou, O. Mondain-Monval and S. Lecommandoux, Langmuir, 2005, 21, 4308–4315 CrossRef.
- J.-S. Jan, S. Lee, C. S. Carr and D. F. Shantz, Chem. Mater., 2005, 17, 4310–4317 CrossRef CAS.
- J. Sun, X. Chen, C. Deng, H. Yu, Z. Xie and X. Jing, Langmuir, 2007, 23, 8308–8315 CrossRef CAS PubMed.
- H. Iatrou, H. Frielinghaus, S. Hanski, N. Ferderigos, J. Ruokolainen and O. Ikkala, et al., Biomacromolecules, 2007, 8, 2173–2181 CrossRef CAS PubMed.
- S. Motala-Timol, D. Jhurry, J. Zhou, A. Bhaw-Luximon, G. Mohun and H. Ritter, Macromolecules, 2008, 41, 5571–5576 CrossRef CAS.
- K. K. Upadhyay, J. F. L. Meins, A. Misra, P. Voisin, V. Bouchaud and E. Ibarboure, et al., Biomacromolecules, 2009, 10, 2802–2808 CrossRef CAS PubMed.
- Y.-F. Huang, S.-C. Lu, Y.-C. Huang and J.-S. Jan, Small, 2014, 10, 1939–1944 CrossRef CAS PubMed.
- J. Huang, G. Habraken, F. Audouin and A. Heise, Macromolecules, 2010, 43, 6050–6057 CrossRef CAS.
- T. Stohr, A.-R. Blaudszun, U. Steinfeld and G. Wenz, Polym. Chem., 2011, 2, 2239–2248 RSC.
- Y.-C. Huang, Y.-S. Yang, T.-Y. Lai and J.-S. Jan, Polymer, 2012, 53, 913–922 CrossRef CAS PubMed.
- B.-Y. Chen, Y.-F. Huang, Y.-C. Huang, T.-C. Wen and J.-S. Jan, ACS Macro Lett., 2014, 3, 220–223 CrossRef CAS.
- Y.-C. Huang and J.-S. Jan, Polymer, 2014, 55, 540–549 CrossRef CAS PubMed.
- H. Kukula, H. Schlaad, M. Antonietti and S. Förster, J. Am. Chem. Soc., 2002, 124, 1658–1663 CrossRef CAS PubMed.
- Č. Koňák, T. Reschel, D. Oupický and K. Ulbrich, Langmuir, 2002, 18, 8217–8222 CrossRef.
- J. H. Jeong, H. S. Kang, S. R. Yang and J.-D. Kim, Polymer, 2003, 44, 583–591 CrossRef CAS.
- B. Nottelet, A. El Ghzaoui, J. Coudane and M. Vert, Biomacromolecules, 2007, 8, 2594–2601 CrossRef CAS PubMed.
- C. Cai, J. Lin, T. Chen and X. Tian, Langmuir, 2009, 26, 2791–2797 CrossRef PubMed.
- Y.-C. Huang, M. Arham and J.-S. Jan, Soft Matter, 2011, 7, 3975–3983 RSC.
- B. A. Clements, V. Incani, C. Kucharski, A. Lavasanifar, B. Ritchie and H. Uludağ, Biomaterials, 2007, 28, 4693–4704 CrossRef CAS PubMed.
- M. Abbasi, H. Uluda, V. Incani, C. Olson, X. Lin and B. A. Clements, et al., Biomacromolecules, 2007, 8, 1059–1063 CrossRef CAS PubMed.
- C. Zhao, X. Zhuang, C. He, X. Chen and X. Jing, Macromol. Rapid Commun., 2008, 29, 1810–1816 CrossRef CAS.
- C. Perrino, S. Lee, S. W. Choi, A. Maruyama and N. D. Spencer, Langmuir, 2008, 24, 8850–8856 CrossRef CAS PubMed.
- J. T. Wilson, V. R. Krishnamurthy, W. Cui, Z. Qu and E. L. Chaikof, J. Am. Chem. Soc., 2009, 131, 18228–18229 CrossRef CAS PubMed.
- S. Saxer, C. Portmann, S. Tosatti, K. Gademann, S. Zuercher and M. Textor, Macromolecules, 2009, 43, 1050–1060 CrossRef.
- M. Abbasi, H. Uluda, V. Incani, M. Yu, C. Hsu and A. Jeffery, Biomacromolecules, 2008, 9, 1618–1630 CrossRef CAS PubMed.
- V. Incani, X. Lin, A. Lavasanifar and H. Uludağ, ACS Appl. Mater. Interfaces, 2009, 1, 841–848 CAS.
- N. Xu, F.-S. Du and Z.-C. Li, J. Polym. Sci., Part A: Polym. Chem., 2007, 45, 1889–1898 CrossRef CAS.
- W. Wang, A. M. McConaghy, L. Tetley and I. F. Uchegbu, Langmuir, 2001, 17, 631–636 CrossRef CAS.
- E. M. Bradbury, C. Crane-Robinson, H. Goldman and H. W. E. Rattle, Biopolymers, 1968, 6, 851–862 CrossRef CAS PubMed.
- K.-H. Liu, B.-R. Chen, S.-Y. Chen and D.-M. Liu, J. Phys. Chem. B, 2009, 113, 11800–11807 CrossRef CAS PubMed.
- Z. Hordyjewicz-Baran, L. You, B. Smarsly, R. Sigel and H. Schlaad, Macromolecules, 2007, 40, 3901–3903 CrossRef CAS.
- A. Harada, S. Cammas and K. Kataoka, Macromolecules, 1996, 29, 6183–6188 CrossRef CAS.
- A. I. Triftaridou, F. Chécot and I. Iliopoulos, Macromol. Chem. Phys., 2010, 211, 768–777 CrossRef CAS.
- J. Gaspard, J. A. Silas, D. F. Shantz and J.-S. Jan, Supramol. Chem., 2009, 22, 178–185 CrossRef.
- Y.-C. Huang, M. Arham and J.-S. Jan, Eur. Polym. J., 2013, 49, 726–737 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra14290j |
| This journal is © The Royal Society of Chemistry 2015 |