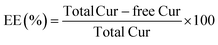Fabrication of zein/quaternized chitosan nanoparticles for the encapsulation and protection of curcumin
Hongshan Liangab,
Bin Zhouab,
Lei Heab,
Yaping Anab,
Liufeng Linab,
Yan Liab,
Shilin Liuab,
Yijie Chenab and
Bin Li*ab
aCollege of Food Science and Technology, Huazhong Agricultural University, Wuhan 430070, China. E-mail: libinfood@mail.hzau.edu.cn; Fax: +86-27-87282966; Tel: +86-27-63730040
bKey Laboratory of Environment Correlative Dietology (Huazhong Agricultural University), Ministry of Education, China
First published on 9th January 2015
Abstract
In this article, we report the successful assembly of nanoparticles (NPs) from a water-soluble chitosan (CS) derivative (N-(2-hydroxyl)propyl-3-trimethyl ammonium chitosan chloride, HTCC) and zein via a low-energy phase separation method. The fabricated NPs were investigated for the first time to encapsulate and protect curcumin (Cur). The particle size and zeta potential of the zein–HTCC NPs varied from 66 to 170 nm and +36.3 to +62.5 mV, respectively. The encapsulation efficiency (EE) was greatly improved to 94.9% after HTCC coating, compared with 85.2% that using zein as a single encapsulant. The microstructure of the NPs was revealed by transmission electron microscopy (TEM). The physicochemical and structural analysis showed that the electrostatic interactions and hydrogen bonds were the major forces responsible for the formation of NPs. The encapsulation forms were evaluated for their efficiency in overcoming Cur's heat and UV sensitivity, which improve the stability about 2.7 fold, 3.5 fold and 2.5 fold when disposed with 60 °C treatment for 30 min, 80 °C treatment for 1 min and ultraviolet radiation for 2 h, respectively at zein–HTCC1 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The results of the stability and DPPH assays indicated that the bioactivity was being protected upon encapsulation. Zein–HTCC NPs are believed to be promising delivery systems for the supplementation or treatment of hydrophobic nutrients or drugs.
1. The results of the stability and DPPH assays indicated that the bioactivity was being protected upon encapsulation. Zein–HTCC NPs are believed to be promising delivery systems for the supplementation or treatment of hydrophobic nutrients or drugs.
1. Introduction
Bioactive compounds have been intensively investigated in recent years for their health-beneficial properties and for potential applications in the fields of pharmaceutics, nutraceuticals, and functional foods.1,2 Among them, polyphenols have attracted many researchers' attention because of their anti-oxidant, anti-inflammatory, and anti-cancer properties.3 Together with some other plant-derived polyphenols, Cur is among the best characterised polyphenols because it is primarily used as a food colourant and more important because it has antioxidant, antibacterial, antifungal, antiviral, anti-inflammatory, antiproliferative, and pro-apoptotic effects.4–6 Unfortunately, the application of Cur has been limited by its poor water solubility and instability, which limits its bioavailability, thus impending its conversion from cooking to clinical applications.7,8 Therefore, the exploitation of Cur as a functional food and nutraceutical ingredient or pharmaceutical compound is feasible only when encapsulated in a delivery system that is capable of stabilizing and protecting it from degradation while preserving its biological activities and enhancing its bioavailability.9Many encapsulation approaches have been applied to increase the water solubility and/or bioavailability of Cur. These include the generation of liposome,10 polymeric micelles,7 emulsion,11 complex,12 NPs,13 etc. Among these approaches, biodegradable polymer NPs offer promising enhanced functional properties for bioactive compounds that are susceptible to degradation during processing and in severe storage conditions, such as heating or ultraviolet radiation.14 Over the past few decades, NP systems based on proteins, including gelatin, collagen, casein, albumin and whey protein have been studied for delivering drugs, nutrients, bioactive peptides and probiotic organisms.15 In most cases, owing to the isoelectric point (pI) of protein, the formulations of NPs are greatly influenced by the pH condition, making the formulations unstable. Therefore, considerable endeavours have been taken in investigating the associative interactions between natural proteins and polysaccharides in order to improve the stability of NPs.16 As an alcohol-soluble protein obtained from corn, zein has attracted widespread interest in delivery systems because of its inherent excellent biocompatibility and biodegradability.17 Zein has been extensively investigated in the encapsulation of bioactive compounds because of its capability to form self-assembled NPs.18,19 It has thus been utilized in food and pharmaceutical applications, such as heparin, gitoxin, fish oil, and the like.20–22 CS, a natural polyaminosaccharide obtained from the N-deacetylation of chitin, with distinctive biological properties, such as non-toxicity, biocompatibility, biodegradability and antimicrobial activity, has been widely used in biomaterial applications.14 However, in neutral and basic environments, the CS molecules lose their charge and precipitation occurs near the pKa of CS (6.5).23 To overcome this drawback and to expand its use, functional groups have been introduced into CS to make it water-soluble.24–27 Among the derivatives of CS, quaternized CS has attracted much attention because of its properties including cationic charge retention at neutral pH, good water solubility, antibacterial activity, mucoadhesivity, enhanced antioxidant activity, and enhanced cellular penetration.28 N-(2-Hydroxyl)propyl-3-trimethylammonium chitosan chloride (HTCC) can be prepared by a relatively easy chemical reaction of CS and 2,3-epoxypropyltrimethyl ammonium chloride (EPTMAC).29 The presence of a positive charge is expected to increase the mucoadhesive nature of CS, which leads to an increased residence time and enhanced bioavailability.30
In the present work, HTCC, which showed higher aqueous solubility than CS in a much broader pH range, was synthesized with a different molecule and used to prepare zein–HTCC NPs. The Cur/zein–HTCC NP delivery system was developed using a liquid–liquid phase separation approach. The characteristics of the Cur encapsulation and protection system have been studied using transmission electron microscopy (TEM), Fourier transform infrared spectroscopy (FT-IR), X-ray diffraction (XRD), fluorescence spectra and DPPH measurements. Additionally, the Cur encapsulation and protection for the zein–HTCC NPs were evaluated. Furthermore, the light stability studies of free Cur and Cur/zein–HTCC1 NPs using the UV absorbance were also investigated. The results illustrated that zein–HTCC NPs greatly increased the water solubility of Cur and had great potential application for bioactive compounds in the field of nutrition and medicine.
2. Materials and methods
2.1. Materials
Zein (Mw = 19k and 22k Da) was purchased from Tokyo Chemical Industry, Co. Ltd. (Tokyo, Japan). CS with a different molecular weight and with 91% deacetylation was supplied by Zhejiang Yuhuan Ocean Biochemistry Co. Ltd. (China). Curcumin (95.0% purity) was purchased from the National Medicine Group Chemical Reagent Co. Ltd. 2,3-Epoxypropyltrimethyl ammonium chloride (EPTMAC) was purchased from Shandong Dongying Chemical Co. Ltd. with a purity of 96%. The other chemicals used were of analytical grade. All the solutions used in the experiments were prepared using ultrapure water through a Millipore (Millipore, Milford, MA, USA) Milli-Q water purification system.2.2. Fourier transform infrared spectroscopy (FT-IR) and 1H-nuclear magnetic resonance (1H NMR) spectroscopy
FT-IR spectra were obtained with a Jasco 4100 series with an attenuated total reflection cell (Jasco Inc., Easton, MO). All samples were prepared as KBr pellets and were scanned against a blank KBr pellet background.The 1H NMR spectra were obtained on a Mercury 400 spectrometer (400 MHz for 1H) in D2O containing a small amount of CD3COOD at 25 °C.
For the pH dependence of the samples' water solubility, the test samples (0.5 g) were dissolved in 1% HAc (50 mL). With stepwise addition of NaOH solution (1 M), the transmittance of the solutions was recorded with a UV-vis spectrophotometer (UV-1100, MAPADA) at 600 nm.
2.3. Synthesis of HTCC
The HTCC was prepared using a method similar to the one reported by Lim and Hudson.31 Briefly, CS (2.0 g, 12.3 mmol) was dispersed in isopropyl alcohol (20.0 mL) and the solution was adjusted to pH 9 by stirring the mixture until the CS was evenly dispersed, and heating the solution to 80 °C. EPTMAC (11.22 g, 73.8 mmol) was dissolved in water and added to the CS suspension at 1 h intervals. After the 6 h reaction, the reaction mixture was precipitated by acetone, washed repeatedly until the solution became neutral, and dissolved in distilled water. The end-product was obtained by freeze drying after a 5 day dialysis.2.4. Degree of quaternization (DQ)
The DQ of the HTCC was measured by titrating the required amount of Cl−1 ions on the HTCC with an aq. AgNO3 solution. DQ is defined as the mol ratio of the bonded EPTMAC per mol of glucosamine calculated from the original mass of CS and its degree of deacetylation (DD).31,32 Thoroughly dried HTCC (0.1000 g) was dissolved in deionized water (100 mL) and conductometrically titrated with a 0.017 M AgNO3 (aq.) solution. Solution conductivities were monitored with Conductometer 644 Metrohm, Swiss. During the titration, the temperature of the solution was kept constant (20.4–20.5 °C) using a water bath.2.5. Molecular weight determination
The average molecular weight (Mw) of the quaternized CS was determined using the gel permeation chromatography (GPC) in conjunction with a multi-angle static light scattering detector (DAWN HELEOS II, WYATT, USA) and a refractive index detector (Optilab T-rEX, WYATT, USA). All the samples were dissolved in an acetate buffer (pH 4.5) and then filtered through nylon syringes filters (450 nm) (Vertical chromatography Co. Ltd. Thailand). The mobile phases, 0.5 M AcOH and 0.5 M AcONa (acetate buffer pH 4.5), were used at a flow rate of 0.6 mL min−1 at 30 °C. The injection volume was 20 μL.2.6. Preparation of zein–HTCC NPs and Cur loading
Zein was dissolved in aqueous ethanol solutions (75% v/v) to obtain a stock solution with a final concentration of 5 mg mL−1. The HTCC solution was prepared by dissolving weighed HTCC powder into water. Then, 1 mL of the zein solution was rapidly mixed with 7 mL of HTCC solution with a different concentration. The solution was stirred vigorously until a single phase was formed, comprising different weight ratios of zein–HTCC at 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 1
2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, respectively.
3, respectively.
For Cur loading, the stock of 20 mg mL−1 Cur prepared with ethanol was first mixed with the zein solution for 60 min. The formulation containing Cur was prepared by adding the above solution dropwise to the HTCC1 solution with magnetic stirring, resulting in different weight ratios of zein–HTCC1 at 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 1
2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, respectively. The finally obtained Cur concentration was 50 μg mL−1.
3, respectively. The finally obtained Cur concentration was 50 μg mL−1.
2.7. Characterizations of NPs
The fluorescence emission spectra of Cur were determined using a Cary Eclipse fluorescence spectrophotometer (Varian Instruments, Walnut Creek, CA) at the excitation wavelength. Cur (10 μg mL−1) was dissolved in ethanol and in 10% v/v ethanol. The excitation wavelength was set at 420 nm, and the emission spectra were ranged from 450 to 700 nm. The slit openings were set at 5 nm for both excitation and emission.
2.8. Encapsulation of Cur
Cur in the percolated solutions was determined using a UV-vis spectrophotometer (UV-1100, MAPADA) at 428 nm. The free Cur was obtained by calculating the Cur content that was ultracentrifuged at 4000 × g for 30 min in a refrigerated centrifuge (TGL-20000cR) with an angular rotor. The encapsulation efficiency (EE) and loading capacity (LC) were defined as the drug content that was entrapped into zein–HTCC NPs and calculated as follows:2.9. Cur protection
To elucidate the effect of encapsulation on the stability of Cur against external severe processing, we compared with remnant content of the free Cur with that of the entrapped Cur in the zein–HTCC NPs after thermal treatment and ultraviolet radiation. Free and encapsulated Cur with a concentration of 10 μg mL−1 shared pasteurization treatment (60 °C, 30 min or 80 °C, 1 min) and ultraviolet radiation (30 W, 254 nm) with 40 cm distance was taken into account for the protective effect. For the Cur encapsulated NPs, ethyl alcohol was added and extracted with the same volume for 4 h, and then evaporated overnight at 40 °C under vacuum.33 The existent Cur was calculated through the absorption value at 428 nm.For light stability studies, the UV absorbance of the free Cur (10 μg mL−1 in 10% v/v ethanol) and Cur/zein–HTCC1 were recorded at 428 nm for 24 h under ambient conditions.
2.10. Radical-scavenging activity using the DPPH method
To guarantee the bioactivity of the encapsulated Cur, the antioxidant activity of Cur was measured according to the DPPH method with minor modification.34,35 Briefly, the scavenging activity assay was carried out by monitoring the absorbance of an ethanolic solution of DPPH (100 μM) at 517 nm in the presence and absence of the test compounds at room temperature with a UV-vis spectrophotometer. The antioxidant activity of Cur was expressed as the percentage of DPPH that was decreased in comparison with that of the control condition (i.e., the testing solution without the presence of Cur) after 30 min preservation in the dark.3. Results and discussion
3.1. Synthesis and characterization of HTCC
In the basic aqueous solution, CS with a different molecular weight (Mw) was coupled with EPTMAC to generate the water-soluble HTCC, in which the amino group of CS was sufficiently nucleophilic to induce the ring-opening of EPTMAC (Scheme 1).23 The Mw and DQ of HTCC are shown in Table 1. The DQ of the HTCC was measured by the conductometric titration of Cl−1 with a 0.017 M aq. AgNO3 solution. The amount of AgNO3 used at the end point equalled the amount of Cl−1 ions presented on the HTCC.31| Sample | Degree of quaternization | Mw |
|---|---|---|
| a HTCC1–HTCC5 represented HTCC with different quaternization degrees and different molecular weights. Data displayed as mean ± SD (n = 3). | ||
| HTCC1 | 0.623 ± 0.02 | 8.708 × 103 |
| HTCC2 | 0.869 ± 0.03 | 1.688 × 105 |
| HTCC3 | 0.879 ± 0.04 | 3.322 × 105 |
| HTCC4 | 0.907 ± 0.03 | 7.051 × 105 |
| HTCC5 | 0.921 ± 0.05 | 1.832 × 106 |
The FT-IR spectra of CS and HTCC were measured with KBr pellets in the range 500–3750 cm−1. In the spectrum of HTCC, the characteristic peak (1568 cm−1) representing NH2 deformation was weakened and two new peaks positioned at 1483 and 2916 cm−1 appeared (Fig. 1a), which were attributed to the bending mode and flex mode of –CH3 in the quaternized ammonium, indicating the introduction of the quaternary ammonium salt group on the CS backbone.24,36 To further confirm the success of the reaction, an 1H NMR analysis of CS and HTCC was performed in CD3COOD–D2O. The NMR spectra of the samples are shown in Fig. 1b and c. As evidence to the reaction, the methyl groups in the quaternary ammonium salt group were observed as a very strong peak at 3.2 ppm.
Fig. 1d shows the pH dependence of the CS and HTCC solutions. At low pH (pH < 6.0), the transmittance was close to 100% not only for the HTCC solution but also for the CS solutions. When the pH increased from 6.0 to 7.0, the transmittance of the CS solution rapidly dropped, and the solution became opaque. In contrast, the transmittance of the HTCC solution did not change. These results illustrate that HTCC has a better solubility in neutral and basic conditions than does CS.32
3.2. Optimization of the formulation
Owing to the cationic properties of HTCC, zein can form NPs with HTCC during the development of novel drug delivery systems. The effects of the preparation parameters on the particle size and the zeta potentials in different formulations are summarized in Tables 2 and 3.| Samples | Size (nm) | PDI | Zeta potential (mV) | EE (%) | LC (%) |
|---|---|---|---|---|---|
| a Z represents the zein NPs without the HTCC coating with the concentration of Cur at 50 μg mL−1. Other samples represent formulations with different mass ratios of zein–HTCC1 with the concentration of Cur at 50 μg mL−1. PDI, polydispersity. EE (%), encapsulation efficiency. LC (%), loading capacity. Data displayed as mean ± SD (n = 3). | |||||
| Z | 134.8 ± 2.9 | 0.25 ± 0.02 | −17.3 ± 1.5 | 85.2 ± 1.2 | 8.5 ± 0.12 |
Z–HTCC1 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
66.4 ± 0.30 | 0.15 ± 0.01 | 36.3 ± 0.6 | 87.7 ± 1.2 | 6.5 ± 0.11 |
Z–HTCC1 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
79.1 ± 0.30 | 0.14 ± 0.02 | 37.9 ± 0.6 | 89.4 ± 2.4 | 5.7 ± 0.16 |
Z–HTCC1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
121.0 ± 1.6 | 0.13 ± 0.01 | 38.3 ± 3.8 | 92.7 ± 1.7 | 4.4 ± 0.08 |
Z–HTCC1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
154.0 ± 2.0 | 0.13 ± 0.03 | 39.0 ± 0.6 | 89.7 ± 2.4 | 2.8 ± 0.08 |
Z–HTCC1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
156.3 ± 1.8 | 0.16 ± 0.05 | 42.8 ± 1.3 | 86.8 ± 4.1 | 2.0 ± 0.10 |
| Samples | Size (nm) | PDI | Zeta potential (mV) | EE (%) | LC (%) |
|---|---|---|---|---|---|
a Samples represent formulations with different Mw of HTCC at the zein–HTCC ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with the concentration of Cur at 50 μg mL−1. PDI, polydispersity. EE (%), encapsulation efficiency. LC (%), loading capacity. Data displayed as mean ± SD (n = 3). 1 with the concentration of Cur at 50 μg mL−1. PDI, polydispersity. EE (%), encapsulation efficiency. LC (%), loading capacity. Data displayed as mean ± SD (n = 3). |
|||||
Z–HTCC2 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
105.1 ± 2.1 | 0.29 ± 0.04 | 48.6 ± 3.1 | 92.9 ± 3.9 | 4.4 ± 0.10 |
Z–HTCC3 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
141.9 ± 7.0 | 0.36 ± 0.06 | 49.1 ± 2.6 | 93.5 ± 2.4 | 4.4 ± 0.06 |
Z–HTCC4 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
158.3 ± 3.1 | 0.48 ± 0.03 | 54.2 ± 4.7 | 93.9 ± 1.2 | 4.4 ± 0.08 |
Z–HTCC5 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
177.0 ± 4.3 | 0.52 ± 0.03 | 62.5 ± 1.2 | 94.9 ± 1.9 | 4.5 ± 0.08 |
The particle size of the Cur-encapsulated zein NPs without HTCC1 coating was 134 nm. After the HTCC1 coating was applied onto the zein NPs, the particle size varied with the weight ratios of zein and HTCC1. With an increase in the HTCC1 ratios, the particle size of the Cur/zein–HTCC1 NPs increased from around 66 to 156 nm. At the zein–HTCC1 ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the particle size was even lower than that of the zein NPs, presumably because the opposite surface charge of zein and HTCC1 could let these two kinds of compounds more close to each other.37 Besides, the NPs had a small PDI (<0.16) except for the Cur/zein NPs, which had a greater PDI of 0.25. The zeta potential of the Cur/zein NPs was −17.3 mV. After being coated by HTCC1, the zeta potential of the NPs became highly positive, in the range +36.3 to +42.8 mV, which slightly augmented with the increase of the HTCC1 concentrations. These observations confirmed that HTCC1 was successfully coated onto the surface of the Cur/zein NPs by electrostatic interactions. The encapsulation efficiency (EE) of the different formulations is depicted in Table 2. The EE of the zein NPs without HTCC1 was around 85.2% and it increased to 92.7% at a zein–HTCC1 ratio of 1
1, the particle size was even lower than that of the zein NPs, presumably because the opposite surface charge of zein and HTCC1 could let these two kinds of compounds more close to each other.37 Besides, the NPs had a small PDI (<0.16) except for the Cur/zein NPs, which had a greater PDI of 0.25. The zeta potential of the Cur/zein NPs was −17.3 mV. After being coated by HTCC1, the zeta potential of the NPs became highly positive, in the range +36.3 to +42.8 mV, which slightly augmented with the increase of the HTCC1 concentrations. These observations confirmed that HTCC1 was successfully coated onto the surface of the Cur/zein NPs by electrostatic interactions. The encapsulation efficiency (EE) of the different formulations is depicted in Table 2. The EE of the zein NPs without HTCC1 was around 85.2% and it increased to 92.7% at a zein–HTCC1 ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Table 2). This could be ascribed to the HTCC1 through electrostatic interactions, resulting in the thick and dense Cur/zein–HTCC1 NPs and therefore an increase in the EE.
1 (Table 2). This could be ascribed to the HTCC1 through electrostatic interactions, resulting in the thick and dense Cur/zein–HTCC1 NPs and therefore an increase in the EE.
The effect of the HTCC Mw on the NPs size, zeta potential, PDI, EE and LC was also investigated (Table 3). The size increased as the Mw of HTCC increased (the ratio of zein–HTCC was kept at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). This phenomenon might be due to longer molecular chains of HTCC with larger Mw entangled with negatively charged zein NPs through ionic interactions that would give rise to a bigger complex. Moreover, the zeta potential increased with the Mw of HTCC. This observation can be easily explained by the stronger electrostatic interactions between HTCC and zein. A higher Mw of HTCC with a higher degree of quaternization was expected to provide more positive charge and compact NPs because a greater number of trimethylammonium groups of HTCC interacted with zein NPs. Moreover, the wide distribution led to an increase in PDI. As shown in Table 3, the higher Mw HTCC led to a higher EE because the longer chain of the HTCC molecule could entrap more Cur.27
1). This phenomenon might be due to longer molecular chains of HTCC with larger Mw entangled with negatively charged zein NPs through ionic interactions that would give rise to a bigger complex. Moreover, the zeta potential increased with the Mw of HTCC. This observation can be easily explained by the stronger electrostatic interactions between HTCC and zein. A higher Mw of HTCC with a higher degree of quaternization was expected to provide more positive charge and compact NPs because a greater number of trimethylammonium groups of HTCC interacted with zein NPs. Moreover, the wide distribution led to an increase in PDI. As shown in Table 3, the higher Mw HTCC led to a higher EE because the longer chain of the HTCC molecule could entrap more Cur.27
3.3. Influence of pH on the zein NPs and zein–HTCC1 NPs
To study the effect of pH on the precipitation kinetics of the zein NPs and zein–HTCC1 NPs, all the samples were studied by dispersing the pre-formed formations in deionized water adjusted to a pH from 2 to 11 using 0.1 N HCl or 0.1 N NaOH. Zein has an isoelectric pH of 6.2 and the particles therefore tend to aggregate at a neutral-basic pH. Precipitation occurred at pH 6 of the zein NPs solution (Fig. 2a). However, the particle size and PDI of the zein–HTCC1 NPs at this pH (Fig. 2b) was relatively small. The zeta potential of the zein NPs was positive at pH < 6, while the zeta potential was negative at pH > 7 (Fig. 2a). Under acidic conditions, the decrease in the zeta potential of the zein–HTCC1 might be due to the stronger ion strength, leading to the charge screening effect. Moreover, at a highly alkaline pH value, the decline in the zeta potential of the zein–HTCC1 NPs was attributed to the augmentation of the zeta potential (negative charge) of the zein NPs. A similar observation was also revealed in other studies.37,38 The particle size of the zein–HTCC1 NPs remained almost constant from pH 3 to 10 (Fig. 2b). The PDI in the whole pH range (2–11), was less than 0.2. Our finding was that the zein–HTCC1 NPs were stable at a broad range of pH, which was an appealing advantage for further applications.3.4. Morphological observation
The morphological observations of the zein NPs, zein–HTCC1 NPs and Cur/zein–HTCC NPs were performed using TEM. Fig. 3a shows a typical size distribution profile of the zein NPs. The TEM micrograph displays that the zein NPs without the HTCC coating were spherical in shape (Fig. 3b). After the zein NPs were coated with HTCC1, the complex formed spherical NPs with a smooth surface and a much smaller and more homogeneous diameter (Fig. 3c and d). The reduced particle size might be contributed by the electrostatic interactions between the zein NPs and the HTCC1 molecules. As shown in Fig. 3e–i, the incorporation of Cur did not cause any morphological changes. When the Mw of HTCC increased, the size of the NPs also increased. The TEM diameter of the NPs was smaller than that obtained from DLS. This phenomenon may be due to the inherent difference in detection of the particle size between DLS and TEM. DLS provides the data of the NPs swollen in solution, whereas that obtained by TEM shows the images of the NPs spread and dried on copper grids coated with a carbon film.39,403.5. FT-IR, XRD and fluorescence spectrum
Fig. 4a shows the spectra of zein, zein–HTCC1 NPs, Cur/zein NPs and Cur/zein–HTCC1 NPs. In the original spectra of zein (Fig. 4a) and HTCC (Fig. 1a), the bands of the hydrogen bonds were at 3441, and 3477 cm−1, respectively. However, after the formation of NPs, a shift of hydrogen bonds occurred, and the peaks were at 3422, 3432, 3438 cm−1 in the spectra of zein–HTCC1 NPs, Cur/zein NPs and Cur/zein–HTCC1 NPs, respectively, thereby suggesting the formation of hydrogen bonds between Cur and zein, as well as between zein and HTCC1. Therefore, the hydrogen bonding among zein, HTCC, and Cur was considered to be one of the major forces facilitating NP formation. The amide I band of zein at 1646 cm−1 showed the prominent C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching and the amide II band at 1549 cm−1 involved C–N stretching. It shifted significantly to 1639 and 1541 cm−1, respectively in Cur/zein NPs, suggesting that the electrostatic interactions were another intermolecular force between Cur and zein. As compared to zein, the bands of the amide I and amide II groups shifted to 1655 and 1542 cm−1, respectively, in zein–HTCC1 NPs, indicating an electrostatic interaction between zein and HTCC1. A further shift of these bands occurred after Cur was encapsulated into NPs. Besides, because both zein and Cur are highly hydrophobic molecules, the hydrophobic interactions could also contribute to the formation of NPs.
O stretching and the amide II band at 1549 cm−1 involved C–N stretching. It shifted significantly to 1639 and 1541 cm−1, respectively in Cur/zein NPs, suggesting that the electrostatic interactions were another intermolecular force between Cur and zein. As compared to zein, the bands of the amide I and amide II groups shifted to 1655 and 1542 cm−1, respectively, in zein–HTCC1 NPs, indicating an electrostatic interaction between zein and HTCC1. A further shift of these bands occurred after Cur was encapsulated into NPs. Besides, because both zein and Cur are highly hydrophobic molecules, the hydrophobic interactions could also contribute to the formation of NPs.
The XRD patterns of the NPs and pure Cur are shown in Fig. 4b. The major characteristic peaks of Cur appeared at 8.90, 12.26, 14.54, 17.24, 23.33, 24.60 and 25.52 degree, indicative of their highly crystalline nature.41 In contrast, zein showed two flatter humps instead of sharp peaks, indicating the amorphous nature of the protein.42 The Cur specific peaks disappeared in all the formations, thus suggesting that Cur in the NPs or in the complex did not exist in a crystalline form, thereby providing additional evidence of encapsulation.
Considering that Cur itself is a fluorescent compound, and the fact that the fluorescence spectrum of a compound is usually affected by its microenvironment, we compared the emission spectrum of Cur in zein–HTCC NPs with that of Cur in ethanol and in 10% v/v ethanol. Cur has very poor water solubility (11 ng mL−1) and is a major factor that limits its in vivo bioavailability. Zein–HTCC1 NPs significantly increased the water solubility of Cur, as evidenced from the evident increase in the Cur fluorescence as compared to the free Cur in 10% v/v ethanol (Fig. 4c). The fluorescence of Cur in the zein–HTCC1 NPs was lower than the fluorescence of Cur in 100% ethanol, which also provides evidence for encapsulation. The emission peak of Cur in ethanol was at 542 nm, whereas the peak shifted to 531 nm when Cur was encapsulated in zein–HTCC1 Cur. This result further confirmed that the microenvironment of Cur was changed upon Cur-encapsulation.4
3.6. Cur protection
As we know, Cur's applications are limited because of its low water solubility and sensitivity to alkaline conditions, thermal treatment, ultraviolet radiation, metallic ions, enzymes, oxygen and ascorbic acid, thus restricting its bioavailability.43 However, pasteurization and radiation sterilization are common technologies in food processing industry. The thermal and ultraviolet light stability of the encapsulated Cur was estimated in comparison to the non-encapsulated Cur (Fig. 5a). After 60 °C treatment for 30 min, 80 °C treatment for 1 min and ultraviolet radiation for 2 h, the unchanged Cur was reduced to 21.9%, 29.2% and 24.5%, respectively for the free Cur (Fig. 5a). When Cur was loaded into the zein NPs, the retention of Cur was 74.5%, 73.5% and 60.4%, respectively (Fig. 5a). Interestingly, the protective effect of the zein–HTCC1 NPs was more obvious. When the weight ratio of zein–HTCC1 was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the Cur preserved could reach upto 79.2%, 77.3% and 60.5%, respectively, when subjected to a 30 min 60 °C treatment, 1 min 80 °C treatment and to a 2 h ultraviolet radiation treatment (Fig. 5a). As compared to the free Cur undergoing the same treatments, the zein–HTCC1 protected Cur content was enhanced by 2.7 fold, 3.5 fold and 2.5 fold, respectively. However, in the same proportion, the protective effect showed no further increase with the increasing Mw (Fig. 5a). The results clearly showed that the stability of the encapsulated Cur at the weight ratio of zein–HTCC1 = 1
1, the Cur preserved could reach upto 79.2%, 77.3% and 60.5%, respectively, when subjected to a 30 min 60 °C treatment, 1 min 80 °C treatment and to a 2 h ultraviolet radiation treatment (Fig. 5a). As compared to the free Cur undergoing the same treatments, the zein–HTCC1 protected Cur content was enhanced by 2.7 fold, 3.5 fold and 2.5 fold, respectively. However, in the same proportion, the protective effect showed no further increase with the increasing Mw (Fig. 5a). The results clearly showed that the stability of the encapsulated Cur at the weight ratio of zein–HTCC1 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, could be protected and improved, which was helpful to broaden the Cur potential applications in the field of nutrition. As can be seen from Fig. 5b, the absorbance of the free Cur decreased rapidly to 50% within 30 min, whereas the absorbance of Cur in the zein–HTCC1 NPs decreased gradually and was seen up to 12 h (Fig. 5c). The light stability of Cur was enhanced dramatically after encapsulation into the zein–HTCC1 NPs.
1, could be protected and improved, which was helpful to broaden the Cur potential applications in the field of nutrition. As can be seen from Fig. 5b, the absorbance of the free Cur decreased rapidly to 50% within 30 min, whereas the absorbance of Cur in the zein–HTCC1 NPs decreased gradually and was seen up to 12 h (Fig. 5c). The light stability of Cur was enhanced dramatically after encapsulation into the zein–HTCC1 NPs.
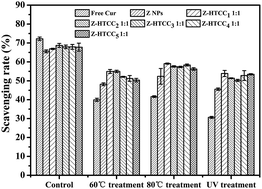
3.7. Antioxidant activity
Antioxidants can scavenge free radicals and maintain the safety, nutritional quality, functionality and palatability of food by retarding the process of lipid peroxidation, which is one of the major reasons for the deterioration of food and pharmaceutical products during processing and storage.44 Cur is a natural compound reported to possess a strong antioxidant activity. In our study, we used the DPPH scavenging method to verify the radical-scavenging ability of the zein–HTCC NPs loaded with Cur. As compared to the untreated control group, the antioxidative effectiveness of the disposed Cur was lower than that of the free Cur (Fig. 6), indicating its instability in the food process, which has also been found in previous studies and which is consistent with the above results.45 The antioxidant activity of the free Cur was about 72.2%, revealing a higher antioxidant activity, whereas the scavenging rate decreased to 39.9%, 41.6% and 30.6% after a 60 °C treatment for 30 min, 80 °C treatment for 1 min and ultraviolet radiation for 2 h, respectively (Fig. 6). When encapsulated into the zein NPs (under the same treatments), the antioxidant activity increased as compared to that of the free Cur, except for the free Cur in the untreated control group (Fig. 6). Comparatively, the Cur/zein–HTCC NPs showed a higher antioxidant activity under the same conditions. Moreover, when the weight ratio of zein–HTCC1 was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the DPPH radical-scavenging ability of the protected Cur improved by 13.3%, 26.9% and 29.0%, as compared to that of the free Cur, when the solutions were pasteurized at low and high temperature and treated with ultraviolet radiation, respectively (Fig. 6). However, when the Mw increased, the antioxidant activity did not enhance any further, which was also in perfect agreement with the protection experiments (Fig. 6). The obtained results confirmed that the loaded Cur still retained its free radical scavenging ability, even after it had been subjected to encapsulation.
1, the DPPH radical-scavenging ability of the protected Cur improved by 13.3%, 26.9% and 29.0%, as compared to that of the free Cur, when the solutions were pasteurized at low and high temperature and treated with ultraviolet radiation, respectively (Fig. 6). However, when the Mw increased, the antioxidant activity did not enhance any further, which was also in perfect agreement with the protection experiments (Fig. 6). The obtained results confirmed that the loaded Cur still retained its free radical scavenging ability, even after it had been subjected to encapsulation.
4. Conclusion
In this work, the zein–HTCC NPs were fabricated successfully as a novel delivery system for Cur, using a low-energy liquid–liquid dispersion method. By coating the Cur/zein NPs with HTCC, the particle size dramatically changed and the zeta potential increased to the highly positive side, depending on the Mw of HTCC. Hydrogen bonding and electrostatic interaction as well as hydrophobic interactions were considered to be the major forces facilitating the formation of NPs. Cur was encapsulated in the zein–HTCC NPs, which improved its solubility and stability, thus preserving its bioactivity during pasteurization and ultraviolet radiation treatment. This has broadened its application in the fields of functional foods and medicine. The worthwhile endeavour elucidated that protein/polysaccharide complexes could effectively solubilize and protect sensitive amphiphilic bioactive compounds. This finding has tremendous implications in the field of nutrition and medicine.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (no. 31071607). The authors would like to express their sincere gratitude to many conveniences offered by the colleagues of Key Laboratory of Environment Correlative Dietology of Huazhong Agricultural University.References
- L. Liang and M. Subirade, J. Phys. Chem. B, 2010, 114, 6707–6712 CrossRef CAS PubMed.
- G. Jayaprakasha, L. J. Rao and K. Sakariah, Food Chem., 2006, 98, 720–724 CrossRef CAS PubMed.
- R. Pegg and F. Shahidi, Food Sci., 2004, 167, 509 Search PubMed.
- H. Yu and Q. Huang, Food Chem., 2010, 119, 669–674 CrossRef CAS PubMed.
- K. Parvathy, P. Negi and P. Srinivas, Food Chem., 2009, 115, 265–271 CrossRef CAS PubMed.
- W. Wu, J. Shen, P. Banerjee and S. Zhou, Biomaterials, 2011, 32, 598–609 CrossRef CAS PubMed.
- M. Esmaili, S. M. Ghaffari, Z. Moosavi-Movahedi, M. S. Atri, A. Sharifizadeh, M. Farhadi, R. Yousefi, J. M. Chobert, T. Haertlé and A. A. Moosavi-Movahedi, LWT--Food Sci. Technol., 2011, 44, 2166–2172 CrossRef CAS PubMed.
- S. Shishodia, M. M. Chaturvedi and B. B. Aggarwal, Curr. Probl. Canc., 2007, 31, 243–305 CrossRef PubMed.
- M. Sessa, R. Tsao, R. Liu, G. Ferrari and F. Donsì, J. Agric. Food Chem., 2011, 59, 12352–12360 CrossRef CAS PubMed.
- S. S. Dhule, P. Penfornis, T. Frazier, R. Walker, J. Feldman, G. Tan, J. He, A. Alb, V. John and R. Pochampally, Nanomedicine, 2012, 8, 440–451 CrossRef CAS PubMed.
- K. Ahmed, Y. Li, D. J. McClements and H. Xiao, Food Chem., 2012, 132, 799–807 CrossRef CAS PubMed.
- W. Xu, W. Jin, C. Zhang, Z. Li, L. Lin, Q. Huang, S. Ye and B. Li, Food Res. Int., 2014, 59, 61–66 CrossRef CAS PubMed.
- M. M. Yallapu, B. K. Gupta, M. Jaggi and S. C. Chauhan, J. Colloid Interface Sci., 2010, 351, 19–29 CrossRef CAS PubMed.
- A. R. Dudhani and S. L. Kosaraju, Carbohydr. Polym., 2010, 81, 243–251 CrossRef CAS PubMed.
- A. Sadeghi, F. Dorkoosh, M. Avadi, P. Saadat, M. Rafiee-Tehrani and H. Junginger, Int. J. Pharm., 2008, 355, 299–306 CrossRef CAS PubMed.
- T. Moschakis, B. S. Murray and C. G. Biliaderis, Food Hydrocolloids, 2010, 24, 8–17 CrossRef CAS PubMed.
- A. R. Patel and K. P. Velikov, Curr. Opin. Colloid Interface Sci., 2014, 19, 450–458 CrossRef CAS PubMed.
- S. Lee, N. S. A. Alwahab and Z. M. Moazzam, Int. J. Pharm., 2013, 454, 388–393 CrossRef CAS PubMed.
- Y. Luo and Q. Wang, J. Appl. Polym. Sci., 2014, 131, 40696 Search PubMed.
- H. J. Wang, Z. X. Lin, X. M. Liu, S. Y. Sheng and J. Y. Wang, J. Controlled Release, 2005, 105, 120–131 CrossRef CAS PubMed.
- L. Muthuselvi and A. Dhathathreyan, Colloids Surf., B, 2006, 51, 39–43 CrossRef CAS PubMed.
- Q. Zhong, H. Tian and S. Zivanovic, J. Food Process. Preserv., 2009, 33, 255–270 CrossRef CAS PubMed.
- A. Wan, Q. Xu, Y. Sun and H. Li, J. Agric. Food Chem., 2013, 61, 6921–6928 CrossRef CAS PubMed.
- S. H. Zhao, X. T. Wu, W. C. Guo, Y. M. Du, L. Yu and J. Tang, Int. J. Pharm., 2010, 393, 269–273 CrossRef PubMed.
- Y. Chang, L. Xiao and Y. Du, Polym. Bull., 2009, 63, 531–545 CrossRef CAS PubMed.
- C. Liu, Y. Tan, C. Liu, X. Chen and L. Yu, J. Ocean Univ. China, 2007, 6, 237–243 CrossRef CAS PubMed.
- S. Shu, L. Sun, X. Zhang, Z. Wu, Z. Wang and C. Li, J. Nanopart. Res., 2011, 13, 3657–3670 CrossRef CAS.
- Y. Cheng, H. Cai, B. Yin and P. Yao, Int. J. Pharm., 2013, 454, 425–434 CrossRef CAS PubMed.
- V. Mourya and N. N. Inamdar, J. Mater. Sci.: Mater. Med., 2009, 20, 1057–1079 CrossRef CAS PubMed.
- T. Sonia and C. P. Sharma, Carbohydr. Polym., 2011, 84, 103–109 CrossRef CAS PubMed.
- S. H. Lim and S. M. Hudson, Carbohydr. Res., 2004, 339, 313–319 CrossRef CAS PubMed.
- Z.-X. Peng, L. Wang, L. Du, S. R. Guo, X. Q. Wang and T. T. Tang, Carbohydr. Polym., 2010, 81, 275–283 CrossRef CAS PubMed.
- A. Kumar and M. Ahuja, Carbohydr. Polym., 2012, 90, 637–643 CrossRef CAS PubMed.
- Y. J. Shang, X. L. Jin, X. L. Shang, J. J. Tang, G. Y. Liu, F. Dai, Y. P. Qian, G. J. Fan, Q. Liu and B. Zhou, Food Chem., 2010, 119, 1435–1442 CrossRef CAS PubMed.
- O. Suwantong, P. Opanasopit, U. Ruktanonchai and P. Supaphol, Polymer, 2007, 48, 7546–7557 CrossRef CAS PubMed.
- W. Li, L. Xiao and C. Qin, J. Mater. Sci., 2010, 45, 5915–5922 CrossRef CAS PubMed.
- Y. Luo, B. Zhang, M. Whent, L. L. Yu and Q. Wang, Colloids Surf., B, 2011, 85, 145–152 CrossRef CAS PubMed.
- S. Podaralla and O. Perumal, AAPS PharmSciTech, 2012, 13, 919–927 CrossRef CAS PubMed.
- G. Wang, B. Yu, Y. Wu, B. Huang, Y. Yuan and C. S. Liu, Int. J. Pharm., 2013, 446, 24–33 CrossRef CAS PubMed.
- K. Zhu, T. Ye, J. Liu, Z. Peng, S. Xu, J. Lei, H. Deng and B. Li, Int. J. Pharm., 2013, 441, 721–727 CrossRef CAS PubMed.
- A. Patel, Y. Hu, J. K. Tiwari and K. P. Velikov, Soft Matter, 2010, 6, 6192 RSC.
- Y. Luo, T. T. Wang, Z. Teng, P. Chen, J. Sun and Q. Wang, Food Chem., 2013, 139, 224–230 CrossRef CAS PubMed.
- B. H. Lee, H. A. Choi, M.-R. Kim and J. Hong, Food Sci. Biotechnol., 2013, 22, 279–282 CrossRef CAS PubMed.
- T. Ak and İ. Gülçin, Chem.-Biol. Interact., 2008, 174, 27–37 CrossRef CAS PubMed.
- E. I. Paramera, S. J. Konteles and V. T. Karathanos, Food Chem., 2011, 125, 913–922 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |