Chemically-driven “molecular logic circuit” based on osmium chromophore with a resettable multiple readout†‡
Anup Kumara,
Megha Chhatwala,
Rinkoo D. Gupta*b and
Satish Kumar Awasthi*a
aChemical Biology Laboratory, Department of Chemistry, University of Delhi, New Delhi-110 007, India. E-mail: skawasthi@chemistry.du.ac.in; satishpna@gmail.com
bFaculty of Life Sciences and Biotechnology, South Asian University, New Delhi-110 021, India. E-mail: rdgupta@sau.ac.in
First published on 8th December 2014
Abstract
The resettable electro-optical identity of an osmium(II) chromophore has been exploited for integrating miniaturised molecular logic circuits under chemical stimulation. The versatile ‘molecular-probe’ yields multiple outputs using selective stimuli and thus allows precise analysis.
Supramolecular receptors,1 capable of chemical recognition,2 have been realised at nano-scale for the meaningful development of molecular gates,3 memory4 and devices.5 However, a commercial molecular device seems to be an Achilles’ heel without integrated logic circuits.6 In this context, the innovative approach of logical transformation of discriminating outputs7 has initiated a leeway for molecular circuit engineering. Importantly, coordination based redox active receptors with multiple outputs are of potential interest for the replication of silicon-transistors at molecular level.
Copper, a micronutrient, can cause severe damages, such as Wilson’s disease, anaemia symptoms, neutropenia, impaired growth, overdose imbalance, etc.8 Fluoride prevents dental cavities and osteoporosis but at toxic levels could cause hypocalcaemia and dental or skeletal fluorosis.9 Importantly, metallic (Cu/Zn) corrosion from fluoridated water during water supply causes the deadly Parkinson and Alzheimer’s diseases.10 In this viewpoint, a chromophore, 1, (Scheme 1) was designed with redox Os(bpy)22+ and a conduit imidazole entity for selective multi-dimensional reversible detection of Cu2+ and F−. Interestingly, discrete chemical information was parallely transduced to multiple spectroscopic and visual outputs to integrate logic circuitry.
The chromophore 1 was synthesized in reasonable yield (46%), characterized by a full battery of physico-chemical techniques (Fig. S1–S5‡) and crystallized with a P21/c space group and monoclinic point group. 1H-NMR of 1 in a DMSO-d6 solution exhibits a peak at δ = 14.47 ppm due to the single imidazole proton, indicating an imine group binding motif.
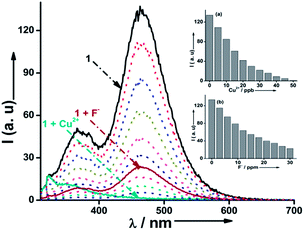
The absorption identity of 1 in acetonitrile exhibits an intense peak at λ = 293 nm (ε = 8.8 × 104 M−1 cm−1) due to a π–π* transition of the ligand-centred (LC) band associated with a couple of broad bands at λ = 509 (ε = 1.4 × 104 M−1 cm−1) and 687 nm (ε = 4.1 × 102 M−1 cm−1), which are assigned to singlet and triplet metal-to-ligand charge transfer (1MLCT and 3MLCT) transitions, respectively.11 Interestingly, 1 displays significant modulation via UV-vis mode on chemical stimulation with Cu2+ and F− in acetonitrile at ambient temperature. For instance, the processing via a 50 ppb concentration of Cu2+, as the input data, produces a bathochromic shift at λ = 293 nm (Δλ = 18 nm) with emergence of a new shoulder at λ = 337 nm, which is associated with a remarkable “turn-off” modulation at λ = 509 and 687 nm (5.0 and 4.5-fold) and a colourimetric change from light brown to colourless in real time (∼15 s). This logical output could be attributed to the generation of oxidative osmium (Os3+) species, as shown from UV-vis and cyclic voltammetry measurements (Fig. 1).12 The output was input selective, as similar inputs viz. Zn2+, Pb2+, Ni2+, Na+, Mn2+, Mg2+, K+, Fe3+, Co2+, Cd2+, Li+, Ca2+, Hg2+ and Fe2+ were inactive (5–10% error, Fig. 2a). The simultaneous processing at multiple wavelengths (λ = 293, 509 and 687 nm) allows the potential option for label-free detection. Importantly, the experiment could be replicated in an aqueous medium with similar efficiency (5–8% error). However, applying other input data, comprising of a 30 ppm concentration of F−, yields entirely different output signals with a MLCT band at λ = 509 nm as well as a shoulder at λ = 325 nm, undergoing a bathochromic shift (Δλ = 45 and 25 nm) along with a colourimetric change from light brown to red in real time (∼15 s) (Fig. 1).
This could be possibly due to the interaction of F− with the acidic protons of the imidazole unit.13 No other tested inputs, viz. I−, Br−, Cl−, NO2−, ClO4−, H3PO4−, AcO−, and CN−, could produce a similar output (Fig. 2b). Note that, probe 1 is only suitable for the sensory action under physiological conditions (in a neutral to slightly acidic medium), as 1 loses selectivity with the OH− ion, and hence can be effectively deployed for biological specimens. Thus, both inputs (Cu2+ and F−) were exclusive and reveal different outputs. Moreover, the parallel addition of a variety of chemical inputs (in the matrix with Cu2+ and/or F−) could not produce any significant change on the obtained output (Fig. S6‡). Importantly, Cu2+ was the predominant input even in case of parallel processing of both Cu2+ and F− via 1. Moreover, the output signals were stationary (Benesi–Hildebrand constant: K1+Cu2+ = 2.74 × 105 M−1 and K1+F− = 4.07 × 104 M−1) and showed no deviation on increasing the concentration/reaction time by up to four times.14 Notably, the evaluated detection limits (DLCu2+ = 1.2 × 10−9 M and DLF− = 2.8 × 10−6 M) are superior so far.15
The repetitive action of a receptor is highly sought after for molecular logic functions. The chemical information written on probe 1 by Cu2+ and F− could be erased (>95% reversibility) by H2O (5 μl in 3 ml) or H+ (5 μl, 10−4 M, 3 ml), respectively (Fig. 3). The reversible tuning through 1 was carried out for 3 cycles and showed significant overall regeneration of up to ∼87%. However, even this minor loss of reversibility could be improved by immobilizing this multitasking receptor on the solid support. Interestingly, the processing of chemical inputs can integrate various complex logic-circuits operating with different outputs.
As shown in Fig. 4, information processing with Cu2+ (input 1) and H2O (input 2) yields an INH (inhibit) logic gate at λ = 509 nm, using a threshold value of A = 0.1 (an absorbance higher/lower than 0.1 will be considered as 0/1, respectively), and an IMP (implication) gate at λ = 293 nm (Fig. S7a and b‡), using a threshold value of A = 0.5 (an absorbance higher/lower than 0.5 will be considered as 0/1, respectively). However, monitoring the information at λ = 554 and 325 nm on processing of F− (input 1) and H+ (input 2) yields coupled INH gates, using A = 0.1 (lower “0” and higher “1”) and 0.4 (lower “1” and higher “0”) as threshold values (Fig. S8a and b‡).
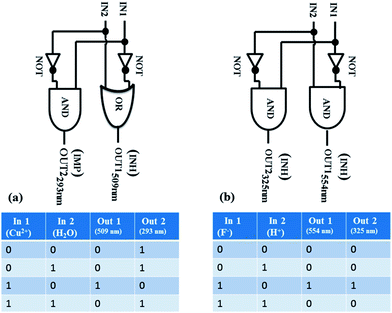 | ||
| Fig. 4 Logic circuit and truth table for the Boolean inputs ((a) Cu2+ and H2O in acetonitrile and (b) F− and H+ in acetonitrile) for 1. | ||
The multi-output generation by a single input could be potentially used for precise and defect-free detection. The recognition propensity of receptor 1 can be monitored via optical as well as electrochemical modes. The receptor 1 exhibits dual fluorescence at λ = 371 and 463 nm on excitation at λ = 293 nm.11 Therefore, the addition of chemical stimuli to 1 triggers dual modulation in fluorescence identity. The processing of 50 ppb of Cu2+ via a fluorogenic mode demonstrates complete quenching of the signal in real time (109-fold at λ = 463 nm and 8-fold at λ = 371 nm). The differential quenching extent with processing of 30 ppm of F− in quick time (6-fold at λ = 463 nm and 371 nm) potentially leads to the selective estimation of each stimulus (Fig. 5). The processing of Cu2+ was predominant in the mixture of analytes with an equal concentration and the written information could be erased (>92% reversibility) by H2O (Cu2+) or H+ (F−) as discussed in the case of the UV-vis mode. The binding constant (Benesi–Hildebrand constant: K1+Cu2+ = 2.32 × 105 M−1 and K1+F− = 3.56 × 104 M−1) and detection limit (DLCu2+ = 1.2 × 10−9 M and DLF− = 2.8 × 10−6 M) evaluated were in agreement with the values obtained by the UV-vis data.
Assuming Cu2+ (In 1), F− (In 2), H2O (In 3), and H+ (In 4) as the four inputs and monitoring the output at λ = 463 nm (threshold value = 50 (a.u), intensity higher/lower than 50 (a.u) will be considered as 1/0, respectively) provide a competent molecular system for the exclusive processing of four distinct chemical informations (Fig. S9‡). Importantly, chromophore 1 yields a distinct response for the exclusive and combinatorial information input following the Boolean logic circuit16 (Fig. 6). The 4-input example, based on fluorescence modulation, is likely to be the first example of this type of integrated logic circuit mimic.
 | ||
| Fig. 6 Molecular logic circuit constructed using four (Cu2+, F−, H2O and H+) inputs and an exclusive output, and the corresponding truth table is shown alongside. | ||
Similar to the optical mode, the sensing mechanism of 1 could be monitored in electrochemical mode also with significant reversibility. The receptor 1 shows one electron transfer reversible redox wave at half wave potential (E1/2) of 0.35 V with peak to peak separation (ΔE) of 75 mV at 300 mV s−1 vs. Ag/AgCl.11 Importantly, the addition of chemical inputs exhibits two-way tuning i.e., anodic shift via Cu2+ (ΔE1/2 = + 0.14 V) and cathodic shift via F− (ΔE1/2 = −0.15 V), supporting the proposed sensory action of 1 (Fig. 7). This discriminating behaviour is exclusive and finds eminent applications in low voltage electrochromic devices.
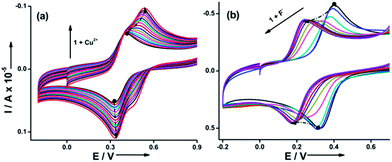 | ||
| Fig. 7 Cyclic voltammograms of 1 (0.97 × 10−3 M, 0.1 mM TBAP, acetonitrile) on processing 50 ppb of Cu2+ (a) and 30 ppb of F− (b) in real time. | ||
Conclusions
In summary, stable duo-optical (chromogenic and fluorogenic) and electrochemical responses of chromophore 1 were exploited for the dual recognition of a cation (Cu2+, 0–50 ppb) and an anion (F−, 0–30 ppm). Moreover, due to its reversible and rapid output/processing under chemical stimulation, an array of logic circuits, viz. two-input/two-output and four-input/one-output, was integrated. The monitoring of the outputs via multiple modes (optical, electrochemical, and visual) provides accurate quantification, widespread utility, and multi-bit information processing at different wavelengths. Importantly, our processor provides label-free detection in a matrix of analytes and allows the retention of unique information with no scope of mixing.Acknowledgements
RDG thanks DST (SERB/F/1424/2013-14) and South Asian University, New Delhi, India for financial assistance. AK and MC thanks UGC and CSIR for SRF. University of Delhi is greatly acknowledged for the technical support.Notes and references
- (a) J. M. Lehn, Science, 1993, 260, 1762–1763 CAS; (b) J. M. Lehn and A. V. Eliseev, Science, 2001, 291, 2331–2332 CrossRef CAS; (c) M. Schmittel and H.-W. Lin, Angew. Chem., Int. Ed., 2007, 46, 893–896 CrossRef CAS PubMed.
- (a) J. M. Lehn, Science, 1985, 227, 849–856 CAS; (b) S. D. Bull, M. G. Davidson, J. M. H. Van Den Elsen, J. S. Fossey, A. T. A. Jenkins, Y. B. Jiang, Y. Kubo, F. Marken, K. Sakurai, J. Zhao and T. D. James, Acc. Chem. Res., 2013, 46, 312–326 CrossRef CAS PubMed; (c) A. Kumar, M. Chhatwal, A. K. Singh, V. Singh and M. Trivedi, Chem. Commun., 2014, 50, 8488–8490 RSC; (d) K. Chen and M. Schmittel, Analyst, 2013, 138, 6742–6745 RSC; (e) K. Chen and M. Schmittel, Chem. Commun., 2014, 50, 5756–5759 RSC.
- (a) A. P. de silva and S. Uchiyama, Nat. Nanotechnol., 2007, 2, 399–410 CrossRef CAS PubMed; (b) T. Gupta and M. E. van der Boom, Angew. Chem., Int. Ed., 2008, 47, 5322–5326 CrossRef CAS PubMed; (c) D. C. Magri, T. P. Vance and A. P. de Silva, Inorg. Chim. Acta, 2007, 360, 751–764 CrossRef CAS PubMed; (d) A. Credi, Angew. Chem., Int. Ed., 2007, 46, 5472–5475 CrossRef CAS PubMed.
- (a) C. Simão, M. Mas-Torrent, J. C. Montenegro, F. Otón, J. Veciana and C. Rovira, J. Am. Chem. Soc., 2011, 133, 13256–13259 CrossRef PubMed; (b) A. Kumar, M. Chhatwal, P. C. Mondal, V. Singh, A. K. Singh, D. A. Cristaldi, R. D. Gupta and A. Gulino, Chem. Commun., 2014, 50, 3783–3785 RSC; (c) U. Pischel, Angew. Chem., Int. Ed., 2010, 49, 1356–1358 CrossRef CAS PubMed; (d) U. Pischel, Aust. J. Chem., 2010, 63, 148–164 CrossRef CAS.
- (a) D. Margulies, G. Melman and A. Shanzer, Nat. Mater., 2005, 4, 768–771 CrossRef CAS PubMed; (b) K. Chen, Q. Shu and M. Schmittel, Chem. Soc. Rev., 2015, 44, 136–160 RSC.
- (a) A. P. de Silva, Nat. Mater., 2005, 4, 15–16 CrossRef CAS; (b) B. Daly, J. Ling and A. P. de Silva, Chem. Sci. Eng., 2014, 8, 240–251 CAS; (c) A. P. de Silva and N. D. McClenaghan, Chem.–Eur. J., 2004, 10, 574–586 CrossRef PubMed.
- (a) J. J. Lavigne and E. V. Anslyn, Angew. Chem., Int. Ed., 2001, 40, 3118–3130 CrossRef CAS; (b) A. Kumar, M. Chhatwal and T. Gupta, Tetrahedron Lett., 2012, 53, 5691–5694 CrossRef CAS PubMed; (c) K. Chen, J. W. Bats and M. Schmittel, Inorg. Chem., 2013, 52, 12863–12865 CrossRef CAS PubMed.
- N. W. Solomons, J. Am. Col. Nutr., 1985, 4, 83–105 CrossRef CAS.
- J. A. Camargo, Chemosphere, 2003, 50, 251–264 CrossRef.
- (a) O. Fejerskov, J. Ekstrand and A. B. Burt, Fluoride in dentistry, Munksgaard, Copenhagen, 2nd edn, 1996 Search PubMed; (b) B. D. Gessner, M. Beller, J. P. Middaugh and G. M. Whitford, N. Engl. J. Med., 1994, 330, 95–99 CrossRef CAS PubMed.
- A. Kumar, A. K. Singh and T. Gupta, Analyst, 2013, 138, 3356–3359 RSC.
- D. Saha, S. Das, S. Karmakar, S. Dutta and S. Baitalik, RSC Adv., 2013, 3, 17314–17334 RSC.
- D. Saha, S. Das, C. Bhaumik, S. Dutta and S. Baitalik, Inorg. Chem., 2010, 49, 2334–2348 CrossRef CAS PubMed.
- H. A. Benesi and J. H. Hildebrand, J. Am. Chem. Soc., 1949, 71, 2703–2707 CrossRef CAS.
- S. Qiu, S. Gao, L. Xie, H. Chen, Q. Liu, Z. Lin, B. Qiu and G. Chen, Analyst, 2011, 136, 3962–3966 RSC.
- G. de Ruiter and M. E. van der Boom, Angew. Chem., Int. Ed., 2012, 51, 8598–8601 CrossRef CAS PubMed.
Footnotes |
| † A dedication to the late Dr Tarkeshwar Gupta. |
| ‡ Electronic supplementary information (ESI) available: X-Ray analysis data for 1, experimental details, characterization, and sensing methodology. CCDC 995811. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4ra14269a |
| This journal is © The Royal Society of Chemistry 2015 |