Properties of some azo-copolyimide thin films used in the formation of photoinduced surface relief gratings
Abstract
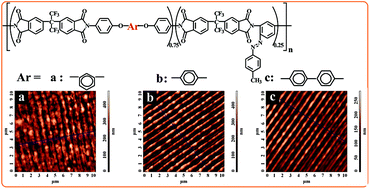
Thin free standing films have been obtained by casting from dimethylacetamide solutions of some azo-copolyimides. The dynamo-mechanical and dielectric properties, and the effect of the chemical structure of polymers on the physical properties are investigated. The incorporation of substituted azobenzene groups and hexafluoroisopropylidene units in the macromolecular chain allowed the patterning of the materials under different irradiation conditions. The azo-copolyimide thin films showed high thermal stability, low dielectric constant, good dynamo-mechanical characteristics and uniform surface relief gratings.

 Please wait while we load your content...
Please wait while we load your content...