One-pot, template-free synthesis of hydrophobic single-crystalline La(OH)3 nanowires with tunable size and their d0 ferromagnetic properties
Abstract
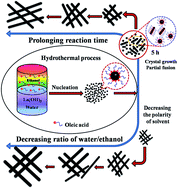
Hydrophobic single-crystalline La(OH)3 nanowires with tunable size have been successfully fabricated by a facile one-pot liquid–solid-solution (LSS) assisted hydrothermal method without any template and their morphology, chemistry and crystal structure were characterized on the nanoscale. Their average diameter and length strongly depend on the reaction time and the selection of solvents, which is due to the Ostwald ripening and oriented attachment mechanisms. XRD patterns and SAED analysis of numerous nanowires show that the La(OH)3 nanowires have a pure hexagonal structure without any impurities. TEM image and HAADF-STEM element mapping analysis indicate that the La(OH)3 nanowires have a uniform size, smooth surface and pure chemical phase. HRTEM images and CBED patterns of individual La(OH)3 nanowires suggest that each nanowire is single crystalline. Magnetic measurements reveal that the La(OH)3 nanowires show a d0 room-temperature ferromagnetic behavior. This study highlights the basic morphological, chemical and structural information for La(OH)3 nanowires, which is critical for their applications in nanodevices and nanoelectronics.

 Please wait while we load your content...
Please wait while we load your content...