Pickering emulsions stabilized by palygorskite particles grafted with pH-responsive polymer brushes
Jia Lua,
Wei Zhoua,
Jing Chena,
Yeling Jina,
Keisha B. Walters*b and
Shijie Ding*a
aCollege of Life Science and Chemical Engineering, Huaiyin Institute of Technology, Huaian, 223003, China. E-mail: shijieding105@yahoo.com; Fax: +86-517-83591165
bDave C. Swalm School of Chemical Engineering, Mississippi State University, 323 President's Circle, Mississippi 39762-9595, Mississippi State, USA. E-mail: kwalters@bagley.msstate.edu; Fax: +1-662-325-2480
First published on 5th January 2015
Abstract
Poly(2-(diethylamino)ethyl methacrylate (PDEAEMA) was grafted from the surface of palygorskite (PAL) particulates via Cu(0) radical polymerization to form PAL–PDEAEMA nanocomposites. The successful grafting was confirmed by Fourier transform infrared (FT-IR), thermal gravimetric analysis (TGA), and elemental analysis (EA). Water (pH 9)/toluene (W/O) Pickering emulsions stabilized by PAL–PDEAEMA particles were prepared. Spherical emulsion droplets with absorbed small PAL–PDEAEMA aggregates on the interface were observed via scanning electron microscopy (SEM), polarizing optical microscopy (POM) and optical microscopy (OM). The size of the emulsion droplets was decreased with the increase of PAL–PDEAEMA particle concentrations, and reached a limited value at 2 wt%. By changing the pH of water (pH < 3), O/W Pickering emulsions were formed by the same PAL–PDEAEMA particles. Moreover, W/O emulsion–de-emulsion–O/W emulsion transitions can be realized simply by adding HCl/NaOH, which can last at least 7 successive cycles. Therefore, a reversible Pickering emulsion system switched by pH can result on the basis of PAL–PDEAEMA particles.
1. Introduction
As early as at the beginning of the last century, Pickering emulsions, which are a kind of emulsion stabilized by solid particles, came into being after the seminal works of Ramsden1 and Pickering2 respectively. Since then, Pickering emulsions have been applied in various fields such as food, pharmaceuticals, cosmetics, agrochemicals and coatings, due to their lower toxicity, easier purification and less expensive costs compared to conventional surfactants. Pickering emulsifiers can be inorganic, organic, or composited particles.3–11 The size, shape and nature of the particulates, most of which are spheres or plate-shaped particles with diameters of nano- to macro-meters, show important effects on the stability of Pickering emulsions.12–14For a stabilized Pickering emulsion, sometimes it demands an instant de-emulsification/re-emulsification and/or phase inversion during the applications. This can be accomplished by environmental stimuli such as pH, temperature, addition of salts, and magnetic fields, among which changing pH is an easy way for handle. Binks investigated the synergistic interaction in emulsions stabilized by a mixture of silica nanoparticles and cationic surfactant, and got the Pickering emulsions with pH responsive behavior by simply lowering the charge of the particles and thereby increasing the hydrophobicity.15 Li and Stöver obtained a pH-responsive Pickering emulsion by the adsorption of small organic molecules (potassium hydrogen phthalate, KHP) with ionic form due to the protonation at low pH onto positively charged alumina coated silica particles.16 Similarly, a pH-sensitive Pickering emulsion was also formed by encapsulation of 8-hydroxyquinoline (8-HQ) which was electrostatically driven adsorption onto silica particles enabled by the protonation of the nitrogen atom in the aromatic ring.17 Dyab prepared the Pickering oil-in-water (O/W) emulsions stabilized solely by partially hydrophobic silica nanoparticles (80% SiOH), which were destabilized by changing the pH of either the silica dispersions before emulsification or that of the freshly prepared emulsions.18 Recently, Wang's group found a simple, reversible emulsion system switched by pH on the basis of chitosan particulates without any hydrophobic modification.19 Perhaps the most extensive investigation on pH responsive behavior of Pickering emulsions has been performed by Armes' group,20–24 who have synthesized a series of lightly cross-linked latex particulate/nanocomposites emulsifiers based on cationic monomers such as 2-(dimethylamino)ethyl methacrylate (DMAEMA), 2-vinylpyridine (2-VP), 4-vinylpyridine (4-VP), 2-(tert-butylamino)ethyl methacrylate (TBAEMA), and 2-(diethylamino)ethyl methacrylate (DEAEMA), respectively.
Palygorskite (PAL) is a kind of clay composed of rod-like crystals with 20–70 nm diameter and several hundred nanometers to several micrometers in length.25 It is a hydrous alumina magnesia silicate with a general chemical formula as Si8O20(Mg,Al,Fe)5(OH)2(OH2)4·4H2O.26 In our previous work,27 we found that without any modification, palygorskite particles can stabilize toluene/water (O/W) emulsions, which can be easily de-emulsified and re-emulsified by simply adding NaOH/HCl to change the pH. Emulsion reversibility can be lasted up to 5 cycles. In this paper, we further our investigation by first report to graft PDEAEMA, which is a well-known, cheap polybase, from the surface of fibrous palygorskite particles (PAL–PDEAEMA) via Cu(0) mediated radical polymerization. Then we used the PAL–PDEAEMA particles as Pickering emulsifier to prepare W/O Pickering emulsion. The effects of some factors, such as particle concentrations, and the pH value of water phase, on the properties of Pickering emulsion were investigated. Moreover, a simple and repeatedly reversible emulsification (W/O)–de-emulsification–emulsification (O/W) Pickering emulsion system based on PAL–PDEAEMA particles was studied. To the best of our knowledge, this kind of investigation has not been reported.
2. Experimental
2.1 Materials
The raw palygorskite (PAL) was obtained from Xuyi Zhongyuan Minerals Co., Ltd (China). Water was passed through a Milli-Q Direct 8 reagent water system. Methanol, ethanol and toluene (all ARs) were obtained from Jiuyi Chemical Reagent Co., Ltd (Shanghai). Tetrahydrofuran (THF), triethylamine (TEA), sodium hydroxide (NaOH) and hydrochloric acid (HCl) (all ARs) were all purchased from Sinopharm Chemical Reagent Co., Ltd (China). Copper wire (99.999%, 0.64 mm diameter) was obtained from Sigma-Aldrich. 2-(Diethylamino)ethyl methacrylate (DEAEMA, 99%), 3-aminopropyltriethoxysilane (APTES, 99%), 1,1,4,7,10,10-hexamethyl triethylenetetramine (HMTETA, 98%), 2-bromoisobutyryl bromide (BIBB, 98%), ethyl 2-bromoisobutyrate (EBIB, 98%) were all purchased from Aladdin Chemistry. All reagents were used as received without further purification.2.2 Preparation of acid-activated PAL
50.0 g of PAL was immersed in 750 mL of 1 mol L−1 HCl solution at room temperature for 24 h under proper mechanical stirring speed, followed by washing with distilled water until the pH nearly reached to 7.0. This acid-activated PAL was then washed in ethanol, centrifuged, vacuum dried at 30 °C for several days, and ground into a size of 200-mesh (<74 μm).2.3 Synthesis of PAL–NH2
18.0 g of acid-activated PAL was dried at 130 °C for 2 h, followed by being placed in 250 mL flask. Then, 200 mL of THF was added. The mixture was stirred for 24 h. Next, 20 mL of APTES was added into the flask and the reaction was performed in an oil bath of 65 °C. After 24 h, the mixture was cooled down and exposed to air, washed extensively with abundant methanol and THF in turn in ultrasonic bath, precipitated with centrifugation and dried under vacuum at 30 °C for 24 h.2.4 Synthesis of PAL–Br macroinitiator
The macroinitiator was obtained according to the following process. 13.0 g of PAL–NH2, 200 mL of THF and 15 mL of TEA were added to a dried 250 mL flask immersed in an ice-water bath. The slurry in the flask was stirred for 0.5 h and 15 mL of 2-bromoisobutyryl bromide (BIBB) was dripped slowly into the slurry. After 5 h, the ice–water bath was removed. The reaction mixture was kept stirring at ambient temperature for 72 h. The product was washed three times with water, twice with methanol and THF in turn, centrifuged and dried under vacuum at room temperature for 24 h.2.5 Cu(0)-mediated radical polymerization of PDEAEMA grafted from the surface of PAL–Br
7.0 mL of DEAEMA monomer was added into a 50 mL flask. After nitrogen was bubbled for 1 h to get rid of the oxygen, 1.2 g of PAL–Br, 7 × 0.5 cm of copper, 40.0 μL (0.20 mmol) of ethyl 2-bromoisobutyrate (EBIB) were successively placed in the flask. Finally, 40.0 μL (0.174 mmol) of HMTETA was added to the mixture, which was stirred in an oil bath at 30 °C under nitrogen atmosphere. After 24 h, the polymer reaction was terminated by being exposed to air and diluted with the solvent. The resultant product was repeatedly washed with methanol and THF in turn, in sonication and centrifugation till no trace of Cu ions and free polymers, and dried under vacuum at room temperature for 24 h.2.6 Preparation of emulsions stabilized by PAL–PDEAEMA particles
A certain concentration of PAL–PDEAEMA particles were first dispersed into 5.0 mL toluene in a glass vessel (inner volume of 15 mL) using a high-intensity ultrasonic vibracell processor (Jeken Ultrasonic Cleaner Limited, PS-40A) with an ultrasonic power of 240 W for 3 min. 5.0 mL water were added into oil dispersions. The emulsions were obtained using a homogenizer (rotor-stator, FSH-2A, Jintan Langbo Instrument Co., China) with a 12 mm head operating at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 3 min. The concentrations of the emulsions were varied from 0.5 wt% to 3.0 wt%, based on the total amount of oil and water (10 g).
000 rpm for 3 min. The concentrations of the emulsions were varied from 0.5 wt% to 3.0 wt%, based on the total amount of oil and water (10 g).
2.7 Characterization
Fourier transform infrared (FT-IR) spectra of palygorskite particles before and after modification were recorded in KBr disks using a Nicolet-5700 FTIR spectrometer (Thermo Fisher Scientific, USA) in the range of 4000–500 cm−1 for 32 scans. Thermal gravimetric analysis (TGA) was conducted on a thermal analysis instrument (STA 409PC, NETZSCH Co., Germany) under nitrogen atmosphere at a balance purge flow rate of 20 cm3 min−1 and a sample purge flow rate of 25 cm3 min−1. Temperature was elevated from 30 °C to 800 °C at a rate of 5 °C min−1. Before TGA running, all the samples were dried in vacuum at 30 °C for 24 h. The C, N and H contents of the samples were performed by element analysis (EA) using Vario EL III (Elementar Analysensysteme GmbH, Germany) under He atmosphere. Mean values in triplicates were obtained with error less than 0.1%. The conductivity of the emulsions was determined using a DDS-307 digital conductivity meter with Pt/Pt black electrodes (INESA Scientific Instrument Co., Ltd, China). The type of emulsions was differentiated according to their conductivities. A high conductivity (>10 μs cm−1) indicated an oil-in-water emulsion, and a low or immeasurable conductivity indicated a water-in-oil emulsion. Emulsion type was also inferred by observing what happened when a drop of each emulsion was added to a volume of pure oil or pure water. Oil continuous (water continuous) emulsions dispersed in oil (water) and remained as drops in water (oil). The pH values were monitored using a pH meter (PHS-3B, Leici, China). Photographs of the emulsions stabilized by PAL–PDEAEMA particles 24 hours after preparation were recorded with a digital camera (DMC-LX5GK, Panasonic, Osaka, Japan). Optical microscopy (OM) of the emulsions involved diluting the samples with its continuous phase and observing it with a PH100-DB500U digital microscope (Phoenix Optical Co., Ltd, China). Images were taken on the connected computer and processed using Nano Measure 1.2 Software to acquire emulsion drop size and its distributions. The mean droplet size was calculated from at least 100 individual measurements of drop diameters. The morphologies of emulsions were observed using a S-3000N scanning electron microscopy (SEM) (Hitachi, Japan). Before SEM observation, the dilute emulsions were stored at −80 °C for 1 h in a MDF-U4186S ultra low temperature refrigerator (Sanyo Co., Ltd, Japan) and then quickly freeze-dried using a lab scale vacuum freeze dryer (Shanghai Yibei Co., Ltd, China) at a cold trap temperature of −50 °C. Micrographs of emulsions after solvent evaporation were obtained using a Leica DM 2500P polarizing optical microscope (POM). The palygorskite clay particles are birefringent but neither toluene nor water is. In polarizing microscope images, birefringent domains are bright, while non-birefringent ones are shown dark under any circumstance. Viscosity of emulsions was characterized using an AR2000ex rheometer (TA Instruments, USA). Shear rate versus viscosity data was collected for emulsion samples over a shear rate range of 0.01–500 s−1. A 60 mm stainless steel plate fixture was used with a 1000 μm gap. All measurements were conducted at 25 °C.3. Results and discussion
3.1 Preparation and characterization of PAL–PDEAEMA particles
The as-received PAL was first treated with acid. This process leads to removal of other impure minerals, such as quartz, feldspars, smectite, carbonates, and partially diminishes the magnesium, iron, and aluminum content of PAL.28 Moreover, the amount of silanol groups (Si–OH) on PAL surface can be further increased during acid treatment.28,29 Acid-activated PAL particles were chemically functionalized with APTES to obtain PAL–NH2. PAL–Br macroinitiator was synthesized by the reaction of PAL–NH2 with 2-bromoisobutyryl bromide. PAL–PDEAEMA particles were prepared by Cu(0)-mediated radical polymerization of monomer DEAEMA. The preparation process of PAL–PDEAEMA particles is shown in Scheme 1.The FT-IR spectroscopy was employed to provide direct identification of chemical groups on PAL, PAL–NH2, PAL–Br, and PAL–PDEAEMA particles (Fig. 1). From Fig. 1a, for unmodified PAL, the band at 3555 cm−1 has been attributed to the symmetric stretching mode of molecular water coordinated to the magnesium at the edges of the channels of palygorskite.30 The band at 1655 cm−1 is attributed to zeolitic water. The band at 1030 cm−1 has been assigned to the asymmetric stretching mode of Si–O–Si. The Si–OH bending band appears at 980 cm−1. From Fig. 1b, amino-modification of PAL is confirmed by a new band at 1558 cm−1, which is attributed to the existence of NH2 on the surface of PAL. Besides, C–H stretching vibration of alkyl occurs at 2935 cm−1. In Fig. 1c, the band at 1536 cm−1 is related to N–H bending vibration of the amide group. The absorption band at 1660 cm−1 ascribing to the stretching vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O is covered up by the bending O–H bands of zeolitic water. Fig. 1 (d) shows the FT-IR spectrum of PAL–PDEAEMA, the prominent peak at 1735 cm−1 is attributable to C
O is covered up by the bending O–H bands of zeolitic water. Fig. 1 (d) shows the FT-IR spectrum of PAL–PDEAEMA, the prominent peak at 1735 cm−1 is attributable to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration of ester group. The absorption bands occurring from 2750–2950 cm−1 are attributed to symmetric stretching vibrations of –NCH2– (for PDEAEMA) of the tertiary amine groups.31 This demonstrates that PDEAEMA was successfully grafted from PAL via the surface-initiated Cu(0) mediated radical polymerization.
O stretching vibration of ester group. The absorption bands occurring from 2750–2950 cm−1 are attributed to symmetric stretching vibrations of –NCH2– (for PDEAEMA) of the tertiary amine groups.31 This demonstrates that PDEAEMA was successfully grafted from PAL via the surface-initiated Cu(0) mediated radical polymerization.
In order to determine the extent of surface modification, the thermogravimetric analyses (TGA) of PAL, PAL–NH2, PAL–Br, PAL–PDEAEMA were carried out. The results in Fig. 2 show that when being heated to 800 °C, the weight losses of PAL, PAL–NH2, PAL–Br, PAL–PDEAEMA are 13.72%, 23.53%, 28.75% and 48.83%, respectively. There is 5.22% difference of the weight loss between PAL–NH2 and PAL–Br, while the difference of weight loss between PAL–Br and PAL–PDEAEMA is 20.08%. TGA curve of PAL–PDEAEMA (Fig. 2d) shows a two-stage weight loss upon heating in nitrogen, in which the second one at the range of 300–600 °C may be assigned to the contribution of PDEAEMA. Obviously, TGA results also testify the successful graft of PDEAEMA from the surface of PAL. Moreover, based on the data of TGA above, the grafting degree of PDEAEMA on palygorskite particles is 55.08% according to the calculation method of literatures.32,33
The results of element analysis (EA) verify the same conclusion as well. As shown in Table 1, the carbon and nitrogen contents have both increased greatly after PAL is modified by APTES (Scheme 1). Effective grafting is demonstrated by the increase in the carbon content from 6.40% to 19.10% and in the nitrogen content from 1.69% to 3.06%. Based on the data from FT-IR, TGA and EA, it can be concluded that the PAL–PDEAEMA particles are successfully prepared.
| Sample | Atomic composition | ||
|---|---|---|---|
| C (%) | N (%) | H (%) | |
| PAL | 0.62 ± 0.01 | 0.28 ± 0.01 | 1.95 ± 0.03 |
| PAL–NH2 | 5.18 ± 0.04 | 1.74 ± 0.01 | 2.36 ± 0.01 |
| PAL–Br | 6.40 ± 0.03 | 1.69 ± 0.08 | 2.53 ± 0.04 |
| PAL–PDEAEMA | 19.10 ± 0.01 | 3.06 ± 0.02 | 4.70 ± 0.04 |
3.2 Pickering emulsions stabilized by PAL–PDEAEMA particles
A certain concentration of PAL–PDEAEMA/oil dispersion was prepared by sonication for 3 min. Then water of pH 9 (if not otherwise specified) and the same volume as oil, was added into the oil dispersions. The emulsions were obtained by homogenization over 3 min. Conductivity values for these emulsions were around 0.5–2.0 μs cm−1, suggesting that a water–oil emulsion was formed. The drop test—placing a drop of the emulsion in water—also gave the same result, with the drop floating on the surface of water.Digital photographs of inclined emulsions at the concentrations of 0.5 wt% and 3.0 wt% are presented and compared (insets in Fig. 4). When the particle concentration was 0.5 wt%, the emulsion showed some degree of mobility; while the particle concentration was 3.0 wt%, the emulsion was solid-like gel that resisted flowing. Viscosity of emulsions at different particle concentrations was measured. As shown in Fig. 4, at a given shear rate, viscosity of emulsions increased with the addition of PAL–PDEAEMA particles. Besides, viscosity of emulsions decreased with increased shear rate, showing a typical shear-thinning flow behavior. And under high shear rate, the viscosity of emulsions was still keeping in relatively high values at high particle concentrations. This may be attributed to the excess PAL–PDEAEMA particles in the continuous phase. The interspaces among emulsion droplets were filled up by those particles, from which the grafted PDEAEMA polymer chains lying on the oil–water interface and in the continuous phase were entangled to prevent the movement of droplets. Consequently, it made the emulsion solidify in the bottle.
Polarized optical microscopy (POM) is a prime tool for examining the crystalline morphology of minerals and inorganic materials, as well as organics.35,36 Recently, this technology has been introduced to inspect the emulsions stabilized by clay.37,38 Since palygorskite is a crystalline hydrated magnesium aluminum silicate composed of rod-like crystals with 20–70 nm diameter and several hundred nanometers to several micrometers in length,25 palygorskite crystal bundles are birefringent.39,40 This feature would not be changed after palygorskite is modified, and can be distinguished qualitatively using a polarizing optical microscope. As shown in Fig. 6, in the picture of polarizing optical micrograph, round emulsion droplets in light specks can be observed, indicating most of palygorskite particles were absorbed at the oil–water interface. In the outside dark area some tiny, bright spots (shown with arrow) also can be seen, suggesting that there are also a few palygorskite particles in the continuous (oil) phase.
 | ||
| Fig. 6 Polarizing optical micrographs (POM) of emulsion stabilized by 1.0 wt% PAL–PDEAEMA particles. | ||
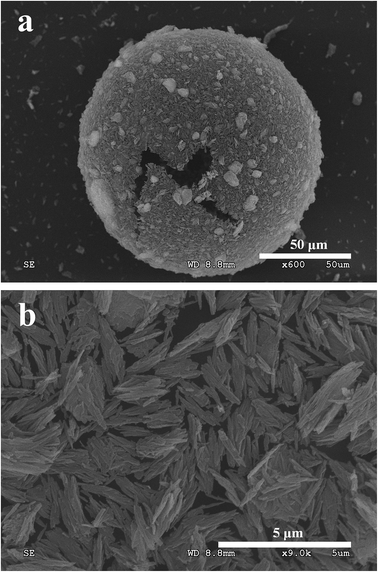
The morphology of emulsions after the freeze-dry procedure is given in Fig. 7. As shown in Fig. 7a, it can be got a clear sight of the PAL–PDEAEMA particles adsorbed at the interface and the formed cavum structure inside due to rapid removal of freeze dried water (ice) under vacuum conditions. At high magnification of SEM (Fig. 7b), it is clearly seen that the PAL–PDEAEMA particles, as a form of small, irregular bundles and nano-rod aggregates, are held at the interface. The original state of PAL–PDEAEMA particles on the oil–water interface has been restored using freeze-dry process.
 | ||
| Fig. 9 (A) Size (average diameters) of emulsion droplets and (B) optical micrographs of emulsions at different pH of water (a) pH 1, (b) 2, (c) 3, (d) 4, (e) 9. The scale bar is 100 μm. | ||
3.2.4 Phase reversibility of emulsions stabilized by PAL–PDEAEMA particles
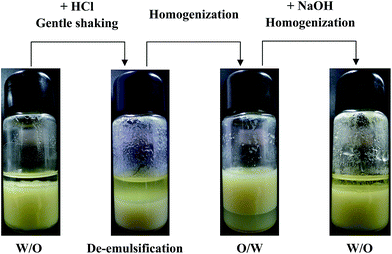
It has been shown above that variation in pH causes phase inversion of the emulsions according to the experiment results. Herein, the reversibility of emulsions stabilized by PAL–PDEAEMA particles was investigated by adding a few drops of 1 M HCl or NaOH to adjust the pH values of the water phase. The transformation of emulsion state switched by pH stimuli is shown in Fig. 11. A stable W/O emulsion with 1.0 wt% PAL–PDEAEMA particles and water of pH 9 was prepared. Next, a given volume (∼0.1 mL) of 1 M HCl was added to the emulsion. De-emulsification took place quickly by gentle shaking for 2–3 min, separated into two distinctive layers. The upper layer is toluene, while the bottom layer is the aqueous suspension containing PAL–PDEAEMA particles. This oil–water two-phase system was then homogenized at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 3 minutes. This time, a stable O/W emulsion was formed. Following this, the same volume of 1 M NaOH as added HCl was put into the emulsion to restore the pH value. A stable W/O emulsion was re-formed after homogenizing. Interestingly, more than seven successive pH cycles could be achieved without a loss of reversibility. Combining with optical micrographs, as shown in Fig. 12, we can conclude that PAL–PDEAEMA composite particles can be used as an excellent recyclable pH-responsive Pickering emulsifier.
000 rpm for 3 minutes. This time, a stable O/W emulsion was formed. Following this, the same volume of 1 M NaOH as added HCl was put into the emulsion to restore the pH value. A stable W/O emulsion was re-formed after homogenizing. Interestingly, more than seven successive pH cycles could be achieved without a loss of reversibility. Combining with optical micrographs, as shown in Fig. 12, we can conclude that PAL–PDEAEMA composite particles can be used as an excellent recyclable pH-responsive Pickering emulsifier.
 | ||
| Fig. 12 Optical micrographs of emulsions in the cycles switched by reversing pH. The scale bar is 100 μm. | ||
The emulsion droplet diameter as a function of the cycle is drawn in Fig. 13. In W/O emulsions, the mean droplet diameters were almost consistent, whereas in O/W emulsions, the mean emulsion drop diameters approximately increased with increasing the number of cycles, especially in the latter several cycles. Obviously, the NaCl concentration would be increased with an increasing number of cycles in this process. The accumulation of salt compresses the electrical double layer, reduces the electrostatic repulsions between particles, and leads to the aggregation or even flocculation of particles.44,45 According to Binks et al.,12 the droplet size of Pickering emulsions increases with particle size. Hence, the O/W emulsion droplets would grow in size, for the NaCl concentration should have more influence on O/W emulsions than on W/O emulsions.
4. Conclusions
We have successfully grafted PDEAEMA from the palygorskite (PAL) particles using Cu(0) mediated radical polymerization, which has been verified by FTIR, TGA, and EA, respectively. A water (pH 9)/toluene (W/O) Pickering emulsion can be stabilized by the prepared PAL–PDEAEMA composite particles. The average size of the emulsion droplets decreased as the particle concentrations were increased, and remained constant after the concentration reached 2.0 wt%. OM, POM and SEM showed spherical emulsion droplets with absorbed small PAL–PDEAEMA aggregates on the interface. As expected, PAL–PDEAEMA particles stabilized W/O emulsion at high pHs (pHs of water in 3–9), whereas O/W emulsions were resulted at low pH (water pH < 3) due to the protonation of amino groups of PDEAEMA. More significantly, by the addition of HCl, W/O emulsions stabilized by PAL–PDEAEMA particulates were de-emulsified instantly, however, after restoring the pH by adding NaOH and re-homogenizing, O/W emulsions were formed. This reversibility can last at least 7 successive cycles. Therefore, PAL–PDEAEMA composite particles can be used as an excellent recyclable pH-responsive Pickering emulsifier.Acknowledgements
The financial supports of National Natural Science Foundation of China (Grant no. 21174046, 51174096) are gratefully acknowledged.References
- W. Ramsden, Proc. R. Soc. London, 1903, 72, 156–164 CrossRef CAS
.
- S. U. Pickering, J. Chem. Soc., 1907, 91, 2001–2021 RSC
.
- B. P. Binks and S. O. Lumsdon, Langmuir, 2000, 16, 2539–2547 CrossRef CAS
.
- R. Aveyard, B. P. Binks and J. H. Clint, Adv. Colloid Interface Sci., 2003, 100, 503–546 CrossRef
.
- N. P. Ashby and B. P. Binks, Phys. Chem. Chem. Phys., 2000, 2, 5640–5646 RSC
.
- Y. Zhu, L. H. Lu, J. Gao, Z. G. Cui and B. P. Binks, Colloids Surf., A, 2013, 417, 126–132 CrossRef CAS PubMed
.
- J. Zhou, X. Qiao, B. P. Binks, K. Sun, M. Bai, Y. Li and Y. Liu, Langmuir, 2011, 27, 3308–3316 CrossRef CAS PubMed
.
- S. Stiller, H. Gers-Barlag, M. Lergenmueller, F. Pflücker, J. Schulz, K. P. Wittern and R. Daniels, Colloids Surf., A, 2004, 232, 261–267 CrossRef CAS PubMed
.
- S. Fujii, D. P. Randall and S. P. Armes, Langmuir, 2004, 20, 11329–11335 CrossRef CAS PubMed
.
- S. Fujii, E. S. Read, B. P. Binks and S. P. Armes, Adv. Mater., 2005, 17, 1014–1018 CrossRef CAS
.
- P. J. Colver, C. A. L. Colard and S. A. F. Bon, J. Am. Chem. Soc., 2008, 130, 16850–16851 CrossRef CAS PubMed
.
- B. P. Binks and S. O. Lumsdon, Langmuir, 2001, 17, 4540–4547 CrossRef CAS
.
- S. Melle, M. Lask and G. G. Fuller, Langmuir, 2005, 21, 2158–2162 CrossRef CAS PubMed
.
- J. Chen, R. Vogel, S. Werner, G. Heinrich, D. Clausse and V. Dutschk, Colloids Surf., A, 2011, 382, 238–245 CrossRef CAS PubMed
.
- B. P. Binks, J. A. Rodrigues and W. J. Frith, Langmuir, 2007, 23, 3626–3636 CrossRef CAS PubMed
.
- J. Li and H. D. H. Stöver, Langmuir, 2008, 24, 13237–13240 CrossRef CAS PubMed
.
- M. F. Haase, D. Grigoriev, H. Moehwald, B. Tiersch and D. G. Shchukin, J. Phys. Chem. C, 2010, 114, 17304–17310 CAS
.
- A. K. F. Dyab, Colloids Surf., A, 2012, 402, 2–12 CrossRef CAS PubMed
.
- H. Liu, C. Wang, S. Zou, Z. Wei and Z. Tong, Langmuir, 2012, 28, 11017–11024 CrossRef CAS PubMed
.
- E. S. Read, S. Fujii, J. I. Amalvy, D. P. Randall and S. P. Armes, Langmuir, 2004, 20, 7422–7429 CrossRef CAS PubMed
.
- D. Dupin, S. P. Armes, C. Connan, P. Reeve and S. M. Baxter, Langmuir, 2007, 23, 6903–6910 CrossRef CAS PubMed
.
- S. Fujii, S. P. Armes, B. P. Binks and R. Murakami, Langmuir, 2006, 22, 6818–6825 CrossRef CAS PubMed
.
- A. J. Morse, D. Dupin, K. L. Thompson, S. P. Armes, K. Ouzineb, P. Mills and R. Swart, Langmuir, 2012, 28, 11733–11744 CrossRef CAS PubMed
.
- A. J. Morse, S. P. Armes, K. L. Thompson, D. Dupin, L. A. Fielding, P. Mills and R. Swart, Langmuir, 2013, 29, 5466–5475 CrossRef CAS PubMed
.
- N. A. Azahari, N. Othman and H. Ismail, Int. J. Polym. Mater., 2012, 61, 1065–1078 CrossRef CAS
.
- J. Chen, S. Ding, Y. Jin and J. Wu, J. Appl. Polym. Sci., 2013, 128, 1779–1784 CAS
.
- J. Lu, X. Tian, Y. Jin, J. Chen, K. B. Walters and S. Ding, Appl. Clay Sci., 2014, 102, 113–120 CrossRef CAS PubMed
.
- H. Chen, Y. Zhao and A. Wang, J. Hazard. Mater., 2007, 149, 346–354 CrossRef CAS PubMed
.
- M. Myriam, M. Suarez and J. M. M. Pozas, Clays Clay Miner., 1998, 46, 225–231 CAS
.
- J. Huang, Y. Liu, Q. Jin, X. Wang and J. Yang, J. Hazard. Mater., 2007, 143, 541–548 CrossRef CAS PubMed
.
- S. Ding, J. A. Floyd and K. B. Walters, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 6552–6560 CrossRef CAS
.
- D. Roy, J. S. Knapp, J. T. Guthrie and S. Perrier, Biomacromolecules, 2007, 9, 91–99 CrossRef PubMed
.
- L. Zhou, W. Yuan, J. Yuan and X. Hong, Mater. Lett., 2008, 62, 1372–1375 CrossRef CAS PubMed
.
- D. E. Tambe and M. M. Sharma, Adv. Colloid Interface Sci., 1994, 52, 1–63 CrossRef CAS
.
- R. A. Carlton, Polarized light microscopy, Springer, New York, 2011, pp. 7–64 Search PubMed
.
- W. Liu, B. Liu and X. Wang, Int. J. Polym. Mater. Polym. Biomater., 2013, 62, 164–171 CrossRef CAS
.
- C. Li, Q. Liu, Z. Mei, J. Wang, J. Xu and D. Sun, J. Colloid Interface Sci., 2009, 336, 314–321 CrossRef CAS PubMed
.
- Y. Cui, M. Threlfall and J. S. van Duijneveldt, J. Colloid Interface Sci., 2011, 356, 665–671 CrossRef CAS PubMed
.
- I. Petkanchin and R. Brückner, Colloid Polym. Sci., 1976, 254, 433–435 CAS
.
- I. Petkanchin, K. Brückner, S. Sokerov and T. Radeva, Colloid Polym. Sci., 1979, 257, 160–165 CAS
.
- J. T. Sun, C. Y. Hong and C. Y. Pan, J. Phys. Chem. C, 2010, 114, 12481–12486 CAS
.
- D. Wang, J. Tan, H. Kang, L. Ma, X. Jin, R. Liu and Y. Huang, Carbohydr. Polym., 2011, 84, 195–202 CrossRef CAS PubMed
.
- Y. Wang, D. Fan, J. He and Y. Yang, Colloid Polym. Sci., 2011, 289, 1885–1894 CAS
.
- S. Cauvin, P. J. Colver and S. A. F. Bon, Macromolecules, 2005, 38, 7887–7889 CrossRef CAS
.
- Y. He, F. Wu, X. Sun, R. Li, Y. Guo, C. Li, L. Zhang, F. Xing, W. Wang and J. Gao, ACS Appl. Mater. Interfaces, 2013, 5, 4843–4855 CAS
.
| This journal is © The Royal Society of Chemistry 2015 |